For none of the actives any PI-103 371935-74-9 reference to COX inhibition was found, and only for compound 9 substructure matches were retrieved with regard to bioactivities other than COX inhibition. It is therefore reasonable to conclude that COX inhibition by compounds 5 and 9 represents a novel finding resulting from our study. We did not perform additional analytical investigations of compound integrity and purity other than those provided by the compound supplier. Therefore, we cannot exclude that the activities measured in the assays might be partially owed to decomposition or oxidation products. Analog compound design and testing will be mandatory. Histone deacetylases regulate the acetylation status of histones and other intracellular substrates. Four classes of HDACs have been identified, three of which are NAD + independent HDACs. The recently discovered class III HDACs are sirtuins. Mammalian sirtuins are homologs of the yeast silent information regulator 2, and are characterized by a unique NAD + -dependent enzymatic activity. Classical HDACs have long been known for their involvement in cancer, including leukemias. Aberrant HDAC activity is commonly observed in leukemia cells, leading to skewed gene expression, increased proliferation, and resistance to apoptosis. HDAC inhibitors, some of which have been available for decades, show antileukemic activity in vitro and in animal models, and thus underwent clinical evaluations, mostly for acute myelogenous leukemia and myelodysplastic syndromes. Overall, these agents are very well tolerated, which makes them particularly suited for treating elderly patients or patients with relevant co-morbidities. However, although the most recent inhibitors, 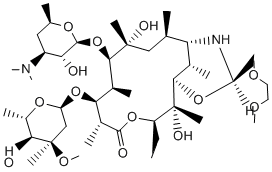 such as vorinostat and romidepsin, appear to be more active than traditional valproic acid, HDAC NVP-BEZ235 915019-65-7 inhibitors alone will rarely induce disease remissions, their benefit being mostly limited to hematological improvements. Thus, strategies to increase their efficacy are warranted. Recently, sirtuins, particularly SIRT1, have also been proposed to play a role in leukemogenesis. SIRT1 was found to be overexpressed in AML and in B-cell chronic lymphocytic leukemia, and downregulated during neutrophil differentiation of acute promyelocytic leukemia cells. It was reported that SIRT1 antagonizes PML-induced cellular senescense. Moreover, increased SIRT1 levels were detected in chemoresistant leukemia cells and in imatinib-resistant chronic myelogenous leukemia cells. The mechanisms invoked to explain SIRT1��s oncogenic activity are mostly related to its role in cell defenses and survival in response to stress. SIRT1 directly deacetylates, and consequently inactivates, p53. Moreover, SIRT1 prevents apoptosis in response to damage or stress by interfering with the activity of the FOXO family of transcription factors, of Bax, Rb, and of E2F1. Sirtuins are virtually unaffected by all HDAC inhibitors currently available. However, numerous small-molecule sirtuin inhibitors have been described, several of which show anticancer activity in preclinical models. Moreover, nicotinamide phosphoribosyltransferase inhibitors, such as FK866, by lowering intracellular NAD + concentrations, deprive sirtuins of their substrate and thus reduce their activity. Indeed, in many instances, pharmacological Nampt inhibition has been shown to recreate the biological consequences of sirtuin obstruction or genetic deletion. In this study, we evaluated sirtuin inhibitors and FK866, either alone or in combination with HDAC inhibitors.
such as vorinostat and romidepsin, appear to be more active than traditional valproic acid, HDAC NVP-BEZ235 915019-65-7 inhibitors alone will rarely induce disease remissions, their benefit being mostly limited to hematological improvements. Thus, strategies to increase their efficacy are warranted. Recently, sirtuins, particularly SIRT1, have also been proposed to play a role in leukemogenesis. SIRT1 was found to be overexpressed in AML and in B-cell chronic lymphocytic leukemia, and downregulated during neutrophil differentiation of acute promyelocytic leukemia cells. It was reported that SIRT1 antagonizes PML-induced cellular senescense. Moreover, increased SIRT1 levels were detected in chemoresistant leukemia cells and in imatinib-resistant chronic myelogenous leukemia cells. The mechanisms invoked to explain SIRT1��s oncogenic activity are mostly related to its role in cell defenses and survival in response to stress. SIRT1 directly deacetylates, and consequently inactivates, p53. Moreover, SIRT1 prevents apoptosis in response to damage or stress by interfering with the activity of the FOXO family of transcription factors, of Bax, Rb, and of E2F1. Sirtuins are virtually unaffected by all HDAC inhibitors currently available. However, numerous small-molecule sirtuin inhibitors have been described, several of which show anticancer activity in preclinical models. Moreover, nicotinamide phosphoribosyltransferase inhibitors, such as FK866, by lowering intracellular NAD + concentrations, deprive sirtuins of their substrate and thus reduce their activity. Indeed, in many instances, pharmacological Nampt inhibition has been shown to recreate the biological consequences of sirtuin obstruction or genetic deletion. In this study, we evaluated sirtuin inhibitors and FK866, either alone or in combination with HDAC inhibitors.
Month: August 2019
Cell division is the process during which a mother cell generates two genetically identical daughter cells
Therefore, the predicted disruption of the PCI-32765 Ubc13-Uev2 heterodimer should be associated with a compromise in tolerance to DNA damage by radiation or radiomimetic drugs in mammalian cells. Additional mechanisms, not explored here but possibly also involved in the chemosensitization caused by compound Ia, could be related to the regulation by Ubc13 of double-strand DNA damage recognition and repair through its interaction with the ubiquitin ligase RNF8. The fact that we have observed inhibition by compound Ia of K63 polyubiquitylation of PCNA only at high concentrations of the compound may suggest either that the compound, although it enters the cells, does not reach the nucleus efficiently, or that K63 polyubiquitylation of PCNA can be catalyzed in mammalian cells by other ubiquitin conjugating enzymes in addition to Ubc13. This may also be the case for K63 polyubiquitylation associated with damage foci in response to DNA double-strand breaks. Indeed, in immunofluorescent cH2AX focus assays, the same batches of compound Ia that inhibited NF-kB activation at low micromolar concentrations only modestly inhibited the maintenance of c-H2AX in ionizing radiation-induced foci. Given the limited effects of compound Ia on both PCNA K63-linked polyubiquitylation and on DNA damage focus formation and resolution, it is possible that the chemosensitization to doxorubicin and etoposide observed in PC-3 and HeLa cells may be better explained by its inhibitory effects on NF-kB signaling. We have observed that compound Ia exerts a direct antitumoral activity in a PC-3 mouse xenograft tumor model. This compound was not directly antiproliferative in vitro for a variety of cell lines tested, but it inhibited the invasiveness of PC-3 cells through extracellular matrix in Boyden chamber experiments, and also inhibited the formation of colonies in 3-dimensional soft-agar cultures. The NF-kB pathway is known to play a prominent role in promoting invasiveness, being constitutively active in PC-3 cells, and thus the observed inhibition of in vitro invasiveness by compound Ia could be one of the consequences of the inhibition of NF-kB activation by this compound. Clonogenicity in soft agar is associated with the capacity of cells for self-renewal, and tends to correlate well with tumorigenicity in vivo. This property, exhibited by distinct cellular subpopulations in some tumors, is not necessarily positively correlated with NF-kB activity, and thus the inhibition by compound Ia of the clonogenicity of PC-3 cells could reflect a requirement 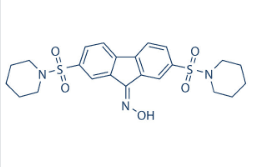 for Ubc13 activity in other pathways regulating the self-renewal capacity of these cells. In any case, the sum of both activities of compound Ia could GDC-0449 explain at least part of the observed direct antitumoral effect. In summary, we have developed specific and potent small molecule antagonists of the Ubc13-Uev1 interaction that inhibit the enzymatic activity of this heterodimer, K63 polyubiquitylation, and we have shown that one of these molecules produces significant effects in the activation of NF-kB by TNF-a, and in invasiveness and clonogenicity in vitro and tumorigenicity of cancer cells in vivo. Based on these activities, we anticipate that tese compounds should be useful to probe other biochemical pathways and cellular processes regulated by K63 polyubiquitylation and to test their effects in relevant models of human pathologies in which these processes are dysregulated. Many anticancer drugs used in the clinic inhibit cell division as tumors are characterized by uncontrolled proliferation.
for Ubc13 activity in other pathways regulating the self-renewal capacity of these cells. In any case, the sum of both activities of compound Ia could GDC-0449 explain at least part of the observed direct antitumoral effect. In summary, we have developed specific and potent small molecule antagonists of the Ubc13-Uev1 interaction that inhibit the enzymatic activity of this heterodimer, K63 polyubiquitylation, and we have shown that one of these molecules produces significant effects in the activation of NF-kB by TNF-a, and in invasiveness and clonogenicity in vitro and tumorigenicity of cancer cells in vivo. Based on these activities, we anticipate that tese compounds should be useful to probe other biochemical pathways and cellular processes regulated by K63 polyubiquitylation and to test their effects in relevant models of human pathologies in which these processes are dysregulated. Many anticancer drugs used in the clinic inhibit cell division as tumors are characterized by uncontrolled proliferation.
Effectively and selectively antagonize the Ubc13-Uev1 interaction and inhibit K63 polyubiquitylation in both yeast and mammalian cells
Our drug development scheme should be applicable to the design of small molecules capable of specifically interfering with many other well-characterized inter or intra-molecular interactions with amenable surfaces. Other non-peptide small molecules that LY2109761 disrupt specific protein-protein interactions have been successfully developed in recent times, and they are beginning to show great promise for the therapy of human cancer. In practical terms, we have developed small molecules, and we have shown that these compounds can be used in combination therapy schemes as antitumoral agents in cultured and animal models of cancer. Specifically, compound Ia sensitizes PC-3 prostate cancer cells to the antiproliferative activity of doxorubicin in cultured cells and it shows direct antitumoral activity in mouse tumor xenografts. A number of mechanisms can be at play to cause increased sensitivities of tumor cells to chemotherapy or radiotherapy, including inhibition of NF-kB, downregulation of transporters of the MDR family or the Akt-mTOR pathway. The evidence provided here suggests that at least two mechanisms may be relevant for the increased sensitivity to doxorubicin caused by compound Ia, namely inhibition of NFk-B activity and compromise of DNA repair. The demonstration that this compound disrupts the interaction between Uev1 and Ubc13 provides a mechanistic explanation for its inhibitory activity on the NF-kB signaling pathway. Recently, it has been shown that another ubiquitin conjugating enzyme, UbcH5, can promote K63 polyubiquitylation, and that NF-kB activation by IL-1b is much more strongly dependent on Ubc13-dependent K63 polyubiquitylation than activation by TNF-a. However, a large body of literature strongly suggests a critical role of Ubc13 and K63 polyubiquitylation in the activation of NF-kB not only by IL-1b but also by TNF-a. In this regard, the chain type of ligand-induced ubiquitylation by cIAP of TNF-R1 complex components has not been determined, and, given the recruitment of Ubc13 by cIAP, it is quite possible that 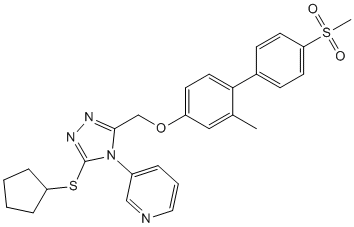 such chains are of the K63 type. Furthermore, mice haploinsuficient for Ubc13 display cell-typespecific defects in chemokine and NF-kB signaling, supporting a critical role of Ubc13 and K63 polyubiquitylation in the activation of NF-kB by different stimuli in vivo, including TNF-a and LPS. Our observations showing that the small molecule antagonist of Ubc13-Uev interactions compound Ia inhibits NF-kB activation by TNF-a would also support a role for Ubc13 in this pathway. Alternative explanations would include the possibility that our compounds inhibit other ubiquitin conjugating enzymes or additional components of the TNF-a signaling BMN673 cascade, which has not been formally ruled out in the present study. On the other hand, it has also been shown that unanchored K63-linked polyubiquitin chains are essential for the activation of the RIG-I pathway in response to viral infection, and that both Ubc13 and Ubc5 are required in this pathway. Therefore, the inhibition of Ubc13 by small compounds could limit the response to viral infections mediated through this pathway. Regarding the role of Ubc13 and K63 polyubiquitylation in DNA damage response, the very high similarity of Uev2 to Uev1, and the computed interaction of compound Ia on the hydrophobic pocket of Ubc13, allows to predict with sufficient confidence that this compound should disrupt also the interaction of Uev2 with Ubc13.
such chains are of the K63 type. Furthermore, mice haploinsuficient for Ubc13 display cell-typespecific defects in chemokine and NF-kB signaling, supporting a critical role of Ubc13 and K63 polyubiquitylation in the activation of NF-kB by different stimuli in vivo, including TNF-a and LPS. Our observations showing that the small molecule antagonist of Ubc13-Uev interactions compound Ia inhibits NF-kB activation by TNF-a would also support a role for Ubc13 in this pathway. Alternative explanations would include the possibility that our compounds inhibit other ubiquitin conjugating enzymes or additional components of the TNF-a signaling BMN673 cascade, which has not been formally ruled out in the present study. On the other hand, it has also been shown that unanchored K63-linked polyubiquitin chains are essential for the activation of the RIG-I pathway in response to viral infection, and that both Ubc13 and Ubc5 are required in this pathway. Therefore, the inhibition of Ubc13 by small compounds could limit the response to viral infections mediated through this pathway. Regarding the role of Ubc13 and K63 polyubiquitylation in DNA damage response, the very high similarity of Uev2 to Uev1, and the computed interaction of compound Ia on the hydrophobic pocket of Ubc13, allows to predict with sufficient confidence that this compound should disrupt also the interaction of Uev2 with Ubc13.
These models reveal that the unique cysteine residue is located at the entrance of the AChE
The almost negligible values of the binding constants suggest a possible lack of hormonal activity. This has been further confirmed by preclinical animal studies using a TTRV30M transgenic mice strain receiving 2.8 mg of iododiflunisal per day during 3 months. The animals did not show significant metabolic disfunctions. However, further preclinical tests are needed to validate these compounds as potential drugs for TTR related amyloidosis. In conclusion, by mimicking the natural interactions between thyroid hormones and TTR and by using diflunisal as a model compound, the biochemical and biophysical data above discussed supports the hypothesis that iodine atoms inserted in TTR binding compounds is a crucial factor for the design of novel PB 203580 152121-47-6 highly potent TTR fibrillogenesis inhibitors that one day become effective drugs for the treatment of TTR-related amyloidosis. Aphids are among the world’s most destructive insect pests of grain crops, vegetables, ornamental plants, and fruit trees. For 150 years the greenbug aphid has been a major pest of small grains. Annual costs for greenbug control in wheat production have been estimated at up to $100 million on the Texas High Plains alone. The soybean aphid causes combined US yield losses and increased production costs that exceed $1 billion. Aphid control relies mainly on a small number of highly toxic anticholinesterases approved by the US Environmental Protection Agency; the threat to agriculture and environmental health is growing. This phenomenon is partly due to an unusual feature of aphid biology. During the aphid-growing season all aphids become female, and are able to produce offspring by maternal cloning in a process known as parthenogenesis. This form of reproduction, with up to 18 asexual generations per growing season, allows aphids to develop resistance rapidly when few effective insecticides are applied repeatedly, as often happens in crops such as soybeans. Acetylcholinesterase is a serine hydrolase vital for regulating the neurotransmitter acetylcholine in mammals, birds, and insects. Current anticholinesterase insecticides such as chlorpyrifos and methamidophos phosphorylate a serine residue at the active site of AChE, thus disabling its function and causing incapacitation. Because this serine residue is also present in mammalian and avian AChEs, use of these insecticides poses serious risks of toxicity to mammals, birds, and beneficial insects such as the honeybee. The US EPA has concluded that such agents can enter the brain of fetuses and young children and may damage the developing nervous system. Controlling aphids in a large field requires insecticides at quantities toxic to mammals and birds. Unintended environmental toxicity is a concern associated with current agents used to Enzalutamide manage these insects. In light of this concern, and the problem of insecticide resistance described above, there is an urgent need for new agents that are both safer and more effective in controlling aphids and related pests. A new concept for insect control is to use an irreversible inhibitor that targets an insect-specific region of an essential protein in the target species. 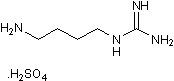 Sequence analyses of various insect proteins identified a cysteine residue that is absent in mammalian and avian AChEs but conserved in the AChEs of aphids and several other insects. This sequence-based finding was consistent with the reports that aphid AChEs were sensitive to sulfhydryl inhibitors. The sequence analysis along with the site-directed mutagenesis and molecular modeling studies on an AChE from amphioxus led to speculations that the cysteine residue conserved in the aphid AChE is located near the top of the active-site gorge and sensitive to sulfhydryl inhibitors and that high affinity bi-functional cholinergic reagents that react transiently with the active site serine and irreversibly with the cysteine residue could be candidates for selective aphicides. The threedimensional models of AChEs in the greenbug and the English grain aphid generated by using terascale computing were reported subsequently.
Sequence analyses of various insect proteins identified a cysteine residue that is absent in mammalian and avian AChEs but conserved in the AChEs of aphids and several other insects. This sequence-based finding was consistent with the reports that aphid AChEs were sensitive to sulfhydryl inhibitors. The sequence analysis along with the site-directed mutagenesis and molecular modeling studies on an AChE from amphioxus led to speculations that the cysteine residue conserved in the aphid AChE is located near the top of the active-site gorge and sensitive to sulfhydryl inhibitors and that high affinity bi-functional cholinergic reagents that react transiently with the active site serine and irreversibly with the cysteine residue could be candidates for selective aphicides. The threedimensional models of AChEs in the greenbug and the English grain aphid generated by using terascale computing were reported subsequently.
Activity of the APEC CH2 ivy knock-out could indicate that this strain also has an additional pliC
In conclusion, this work is the first to demonstrate the involvement of a lysozyme inhibitor in bacterial virulence. Although findings from the APEC – chicken model system studied in this work cannot be simply extrapolated to other pathogen – host interactions, the wide distribution of different types of lysozyme inhibitors in bacteria suggests that these molecules have evolved as virulence factors or effectors of commensal interactions in a wide range of bacteria. This finding may also open Temozolomide perspectives for new avenues for the development of antibacterial drugs, for example by designing compounds that can neutralize bacterial lysozyme inhibitors, thus rendering them more sensitive to the host lysozymes. Hepatic fibrosis is a reversible wound-healing response characterized by the accumulation of extracellular matrix in response to acute or chronic liver injury. Perpetuation of the fibrotic reaction can lead to end-stage liver disease, cirrhosis, and hepatocellular carcinoma, whose incidence is increasing worldwide. The activation and proliferation of hepatic stellate cells has been identified as a critical event in the development of hepatic fibrosis. Activated HSCs are highly contractile and express a-smooth muscle actin and ECM. They are a key target for anti-fibrotic therapies because these cells are the primary source of ECM in injured livers. The Notch signaling pathway is a highly conserved signal transduction mechanism. It is essential to normal embryonic development, cellular proliferation, specification, and differentiation. Four Notch receptors and five ligands have been identified in mammals. Notch signaling is activated through an interaction of a Notch receptor with a ligand expressed on adjacent cells leading to proteolytic cleavages of Notch receptor. The cleavage step catalyzed by the  csecretase complex results in the release of the Notch intracellular domain. The NICD then moves to the nucleus, where it interacts with CSL and Mastermind to activate transcription of downstream target genes such as Hes1, HRT, Deltes-1, Meltrin-b, and the Notch receptors themselves. Notch signaling is essential to the regulation of cell differentiation, and aberrant activation of this pathway is implicated in the pathogenesis of several malignancies. Increasing numbers of studies have reported that Notch signaling is involved in human fibrotic diseases. However, the role of Notch signaling in liver fibrosis has not been fully investigated. Previous studies have indicated that all 4 receptors are expressed in the adult liver, with no significant differences in the levels of Notch1, 2, and 4 mRNA between normal and Y-27632 dihydrochloride diseased livers. However, the expression of Notch3 and Jagged1 protein has been found to be significantly up-regulated in diseased liver tissue. Recent research has found the mRNA of Notch receptors to be present in freshly isolated rat HSCs, which displayed no protein synthesis of Notch ligands. However, the amount of Jagged1 protein increased while isolated HSCs developed into myofibroblast-like cells. Based on these studies, we hypothesize that Notch signaling might be involved in liver fibrogenesis. In the present study, we investigated the role of Notch signaling during the process of liver fibrosis and clarified its mechanism. Our results demonstrated that Notch signaling is activated in hepatic fibrosis induced by CCl4 and that blocking Notch signaling using c-secretase inhibitor can significantly attenuate liver fibrosis. These results suggest that selective interruption of Notch signaling might be a novel anti-fibrotic strategy in hepatic fibrosis. In this study, we show that Notch signaling is markedly activated in a rat model of liver fibrosis induced by CCl4.
csecretase complex results in the release of the Notch intracellular domain. The NICD then moves to the nucleus, where it interacts with CSL and Mastermind to activate transcription of downstream target genes such as Hes1, HRT, Deltes-1, Meltrin-b, and the Notch receptors themselves. Notch signaling is essential to the regulation of cell differentiation, and aberrant activation of this pathway is implicated in the pathogenesis of several malignancies. Increasing numbers of studies have reported that Notch signaling is involved in human fibrotic diseases. However, the role of Notch signaling in liver fibrosis has not been fully investigated. Previous studies have indicated that all 4 receptors are expressed in the adult liver, with no significant differences in the levels of Notch1, 2, and 4 mRNA between normal and Y-27632 dihydrochloride diseased livers. However, the expression of Notch3 and Jagged1 protein has been found to be significantly up-regulated in diseased liver tissue. Recent research has found the mRNA of Notch receptors to be present in freshly isolated rat HSCs, which displayed no protein synthesis of Notch ligands. However, the amount of Jagged1 protein increased while isolated HSCs developed into myofibroblast-like cells. Based on these studies, we hypothesize that Notch signaling might be involved in liver fibrogenesis. In the present study, we investigated the role of Notch signaling during the process of liver fibrosis and clarified its mechanism. Our results demonstrated that Notch signaling is activated in hepatic fibrosis induced by CCl4 and that blocking Notch signaling using c-secretase inhibitor can significantly attenuate liver fibrosis. These results suggest that selective interruption of Notch signaling might be a novel anti-fibrotic strategy in hepatic fibrosis. In this study, we show that Notch signaling is markedly activated in a rat model of liver fibrosis induced by CCl4.