In conclusion, this work is the first to demonstrate the involvement of a lysozyme inhibitor in bacterial virulence. Although findings from the APEC – chicken model system studied in this work cannot be simply extrapolated to other pathogen – host interactions, the wide distribution of different types of lysozyme inhibitors in bacteria suggests that these molecules have evolved as virulence factors or effectors of commensal interactions in a wide range of bacteria. This finding may also open Temozolomide perspectives for new avenues for the development of antibacterial drugs, for example by designing compounds that can neutralize bacterial lysozyme inhibitors, thus rendering them more sensitive to the host lysozymes. Hepatic fibrosis is a reversible wound-healing response characterized by the accumulation of extracellular matrix in response to acute or chronic liver injury. Perpetuation of the fibrotic reaction can lead to end-stage liver disease, cirrhosis, and hepatocellular carcinoma, whose incidence is increasing worldwide. The activation and proliferation of hepatic stellate cells has been identified as a critical event in the development of hepatic fibrosis. Activated HSCs are highly contractile and express a-smooth muscle actin and ECM. They are a key target for anti-fibrotic therapies because these cells are the primary source of ECM in injured livers. The Notch signaling pathway is a highly conserved signal transduction mechanism. It is essential to normal embryonic development, cellular proliferation, specification, and differentiation. Four Notch receptors and five ligands have been identified in mammals. Notch signaling is activated through an interaction of a Notch receptor with a ligand expressed on adjacent cells leading to proteolytic cleavages of Notch receptor. The cleavage step catalyzed by the 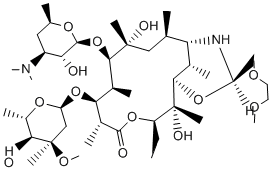 csecretase complex results in the release of the Notch intracellular domain. The NICD then moves to the nucleus, where it interacts with CSL and Mastermind to activate transcription of downstream target genes such as Hes1, HRT, Deltes-1, Meltrin-b, and the Notch receptors themselves. Notch signaling is essential to the regulation of cell differentiation, and aberrant activation of this pathway is implicated in the pathogenesis of several malignancies. Increasing numbers of studies have reported that Notch signaling is involved in human fibrotic diseases. However, the role of Notch signaling in liver fibrosis has not been fully investigated. Previous studies have indicated that all 4 receptors are expressed in the adult liver, with no significant differences in the levels of Notch1, 2, and 4 mRNA between normal and Y-27632 dihydrochloride diseased livers. However, the expression of Notch3 and Jagged1 protein has been found to be significantly up-regulated in diseased liver tissue. Recent research has found the mRNA of Notch receptors to be present in freshly isolated rat HSCs, which displayed no protein synthesis of Notch ligands. However, the amount of Jagged1 protein increased while isolated HSCs developed into myofibroblast-like cells. Based on these studies, we hypothesize that Notch signaling might be involved in liver fibrogenesis. In the present study, we investigated the role of Notch signaling during the process of liver fibrosis and clarified its mechanism. Our results demonstrated that Notch signaling is activated in hepatic fibrosis induced by CCl4 and that blocking Notch signaling using c-secretase inhibitor can significantly attenuate liver fibrosis. These results suggest that selective interruption of Notch signaling might be a novel anti-fibrotic strategy in hepatic fibrosis. In this study, we show that Notch signaling is markedly activated in a rat model of liver fibrosis induced by CCl4.
csecretase complex results in the release of the Notch intracellular domain. The NICD then moves to the nucleus, where it interacts with CSL and Mastermind to activate transcription of downstream target genes such as Hes1, HRT, Deltes-1, Meltrin-b, and the Notch receptors themselves. Notch signaling is essential to the regulation of cell differentiation, and aberrant activation of this pathway is implicated in the pathogenesis of several malignancies. Increasing numbers of studies have reported that Notch signaling is involved in human fibrotic diseases. However, the role of Notch signaling in liver fibrosis has not been fully investigated. Previous studies have indicated that all 4 receptors are expressed in the adult liver, with no significant differences in the levels of Notch1, 2, and 4 mRNA between normal and Y-27632 dihydrochloride diseased livers. However, the expression of Notch3 and Jagged1 protein has been found to be significantly up-regulated in diseased liver tissue. Recent research has found the mRNA of Notch receptors to be present in freshly isolated rat HSCs, which displayed no protein synthesis of Notch ligands. However, the amount of Jagged1 protein increased while isolated HSCs developed into myofibroblast-like cells. Based on these studies, we hypothesize that Notch signaling might be involved in liver fibrogenesis. In the present study, we investigated the role of Notch signaling during the process of liver fibrosis and clarified its mechanism. Our results demonstrated that Notch signaling is activated in hepatic fibrosis induced by CCl4 and that blocking Notch signaling using c-secretase inhibitor can significantly attenuate liver fibrosis. These results suggest that selective interruption of Notch signaling might be a novel anti-fibrotic strategy in hepatic fibrosis. In this study, we show that Notch signaling is markedly activated in a rat model of liver fibrosis induced by CCl4.