Of the genes regulated by both treatments, the majority was regulated in the same direction. Whether this overlap reflects an overall beneficial metabolic outcome in common to both Albaspidin-AA treatments or more direct effects remain to be 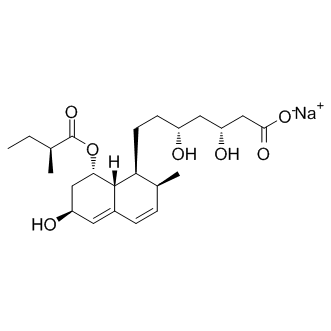 elucidated. Several of the transcripts that we identified to be regulated by FGF21 treatment, encode secreted proteins. From a biomarker perspective, these would allow for the development of blood-based protein detection assays to measure FGF21 target engagement, which would be less invasive in a clinical Folinic acid calcium salt pentahydrate setting than adipose tissue biopsies. Over 300 such transcripts were identified following acute treatment with FGF21. The plasma levels of 5 of these proteins for which there were commercially available antibodies were determined in a validation study and the FGF21-induced changes in plasma agreed with their gene expression changes in IWAT for at least 2 of them. Three other proteins were tested in the circulation but they did not show dose-dependent regulation using the available reagents, although there was a change in plasma protein levels for all three proteins at the highest dose of FGF21, in agreement with the RNA profiling data. Further studies are required to validate the additional potential secreted proteins once reagents become available. However, one secreted protein of relevance to these studies is adiponectin, which has recently been described as having a key role in mediating FGF21��s beneficial metabolic effects in mice. In the present study, the mRNA of this gene in WAT was not altered following FGF21 treatment, perhaps reflecting the high basal level of expression in this tissue. Consistent with this observation, we also could not detect an increase in secreted adiponectin in either the culture medium of 3T3L1 adipocytes or in the circulation of mice treated in vivo. In contrast, we did recapitulate published data with rosiglitazone in parallel experiments, where secreted adiponectin levels were significantly increased. Moreover, the absence of increases in adiponectin mRNA and protein levels could not be attributed to a lack of FGF21 effect, since we observed a robust increase in Erk phosphorylation, a proximal TE biomarker, both in vitro and in vivo. Further studies are required to determine whether these differences reflect nuanced experimental conditions such as experimental design, cell / mouse sources or reagent sources. In the current series of experiments, the impact of FGF21 on BAT gene expression was more robust than in the WAT; while this is consistent with the increased oxidation of glucose observed in db/db mice following PEG30-FGF21 treatment, further experiments are needed to fully elucidate any tissuespecific differences between the metabolic effects of FGF21 on BAT and WAT. In summary, transcriptomic and phosphoproteomic profiling analyses have been performed in mouse adipose tissues in vivo and 3T3L1 adipocytes, respectively, following acute FGF21 treatment to further elucidate the downstream pathways affected. In addition to confirming a number of previously reported signaling cascades, our studies also uncovered several novel transcriptional and post-translational events that warrant further investigation. Several key biomarkers were identified that may serve not only as clinical readouts of FGF21 target engagement but might also help elucidate the mechanisms of action in this primary target tissue that mediate FGF21’s beneficial metabolic effects. Specifically, we identified distinct transcriptional effects of FGF21 in brown and white adipose tissue. This opens up a window for further studies to investigate both the therapeutic benefits of FGF21 and to compare the metabolic differences between these distinct adipose tissue types.
elucidated. Several of the transcripts that we identified to be regulated by FGF21 treatment, encode secreted proteins. From a biomarker perspective, these would allow for the development of blood-based protein detection assays to measure FGF21 target engagement, which would be less invasive in a clinical Folinic acid calcium salt pentahydrate setting than adipose tissue biopsies. Over 300 such transcripts were identified following acute treatment with FGF21. The plasma levels of 5 of these proteins for which there were commercially available antibodies were determined in a validation study and the FGF21-induced changes in plasma agreed with their gene expression changes in IWAT for at least 2 of them. Three other proteins were tested in the circulation but they did not show dose-dependent regulation using the available reagents, although there was a change in plasma protein levels for all three proteins at the highest dose of FGF21, in agreement with the RNA profiling data. Further studies are required to validate the additional potential secreted proteins once reagents become available. However, one secreted protein of relevance to these studies is adiponectin, which has recently been described as having a key role in mediating FGF21��s beneficial metabolic effects in mice. In the present study, the mRNA of this gene in WAT was not altered following FGF21 treatment, perhaps reflecting the high basal level of expression in this tissue. Consistent with this observation, we also could not detect an increase in secreted adiponectin in either the culture medium of 3T3L1 adipocytes or in the circulation of mice treated in vivo. In contrast, we did recapitulate published data with rosiglitazone in parallel experiments, where secreted adiponectin levels were significantly increased. Moreover, the absence of increases in adiponectin mRNA and protein levels could not be attributed to a lack of FGF21 effect, since we observed a robust increase in Erk phosphorylation, a proximal TE biomarker, both in vitro and in vivo. Further studies are required to determine whether these differences reflect nuanced experimental conditions such as experimental design, cell / mouse sources or reagent sources. In the current series of experiments, the impact of FGF21 on BAT gene expression was more robust than in the WAT; while this is consistent with the increased oxidation of glucose observed in db/db mice following PEG30-FGF21 treatment, further experiments are needed to fully elucidate any tissuespecific differences between the metabolic effects of FGF21 on BAT and WAT. In summary, transcriptomic and phosphoproteomic profiling analyses have been performed in mouse adipose tissues in vivo and 3T3L1 adipocytes, respectively, following acute FGF21 treatment to further elucidate the downstream pathways affected. In addition to confirming a number of previously reported signaling cascades, our studies also uncovered several novel transcriptional and post-translational events that warrant further investigation. Several key biomarkers were identified that may serve not only as clinical readouts of FGF21 target engagement but might also help elucidate the mechanisms of action in this primary target tissue that mediate FGF21’s beneficial metabolic effects. Specifically, we identified distinct transcriptional effects of FGF21 in brown and white adipose tissue. This opens up a window for further studies to investigate both the therapeutic benefits of FGF21 and to compare the metabolic differences between these distinct adipose tissue types.