SAP97 has been shown to interact with the C-terminus of NMDA and a-amino-3-hydroxy-5-methyl-4isoxazolepropionic acid receptors. Recently, SAP97 has also been implicated in trafficking of NMDARs at the cell surface by sorting them through an unconventional secretory pathway. In this study, we report a novel role for Lomitapide Mesylate DlgS97 and SAP97 in the development of Cinoxacin tolerance to ethanol. By testing multiple independently isolated dlg1 alleles, we determined that DlgS97 is required for the development of rapid ethanol tolerance in Drosophila. We further demonstrate that hypomorphic mutants of Nmdar1, encoding the Drosophila NMDA receptor 1 subunit, or Camguk/Caki, encoding the homolog of CASK that interacts with the L27 domain of DlgS97, exhibit reduced ethanol tolerance. Co-immunoprecipitation of dNR1 and DlgS97 confirmed a physical interaction of DlgS97 and NMDARs. We propose that this interaction is of functional relevance to ethanol tolerance development. Lastly, we show that adult mice with reduced expression of SAP97 display deficits in the development of rapid tolerance to the sedative/hypnotic effects of ethanol, measured using regain of the loss of righting response. Unless otherwise stated, adult male flies were used for all experiments. Flies were collected under CO2 anesthesia 2�C3 days before experiments. Ethanol sensitivity and tolerance were quantified using the inebriometer, an apparatus that measures the loss of postural control with increasing time of exposure to ethanol vapor of a sample population of flies. To quantify ethanol tolerance, a sample of,120 flies was exposed to ethanol vapor at a relative flow rate of 60/40 ethanol vapor/humidified air for 30 min, transferred to large vials containing fly food, and allowed to recover from sedation for an additional 3.5 hr. In parallel, a second, control sample otherwise identically treated was exposed to humidified air without ethanol at an equivalent total flow rate and allowed to recover. Following recovery, each pair of ethanol pre-exposed and control samples were assayed using side-by-side inebriometers. Placenta is a highly specialized temporary organ responsible for the normal progression of pregnancy in mammals. Defects in implantation, placental development and maturation lead to complications in pregnancy and newborns. Aberrant placental expression of apoptosis and inflammation-related genes in early pregnancy was found in recurrent miscarriage and hydatidiform mole samples. Altered placental transcription of metabolic regulatory genes has been associated to affected fetal growth and maternal pregnancy complications such as preeclampsia and gestational diabetes mellitus. Differential expression of some genes is common to several pregnancy complications, serving as biomarkers for malfunctioning placenta. Despite the great importance of placenta in mediating the rapid physiological changes in pregnancy, data on the temporal dynamics of human placental gene expression are limited. Large-scale differences in DNA 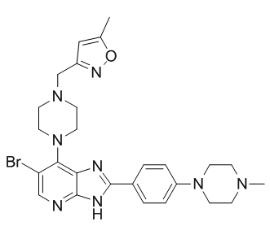 methylation levels between first, second and third trimesters support the gestational-age dependent function of placental genes. So far, only three published studies focus on gene expression in term placenta compared to either early or mid-gestation pregnancy, or both. All studies agree that global gene expression undergoes a profound transformation at the end of pregnancy, affecting up to 25% of placental transcriptome and involving coordinated up- or downregulation of functionally linked loci to achieve rapid changes in placental function.
methylation levels between first, second and third trimesters support the gestational-age dependent function of placental genes. So far, only three published studies focus on gene expression in term placenta compared to either early or mid-gestation pregnancy, or both. All studies agree that global gene expression undergoes a profound transformation at the end of pregnancy, affecting up to 25% of placental transcriptome and involving coordinated up- or downregulation of functionally linked loci to achieve rapid changes in placental function.
Month: June 2019
Members of the cullin family are covalently modified by NEDD8 where ubiquitating ligase functioned
In SILAC, normalization was performed using the original mixture of the cells at a 1:1 ratio and reaching 100% labeling efficiency for both cell populations. The limitation of selecting a threshold of expression to consider proteins to be differentially expressed requires a followup validation analysis for key data. Verification of changes by two independent analytical methods, and using independent in vitro Cinoxacin strategies and clinical material provided confidence that the experimental design permitted significant changes in abundance to be validated. The limited correlation between transcript and protein expression at their steady state was similar to the 0.28 previously reported in pancreatic cells. This could be attributed to the wider range of ratios of expression measured by gene arrays while the majority of the SILAC ratios were in the low range. SILAC ratios were more limited due to the internal labelling and the characteristic 1:1 mixture of the protein extracts analyzed. The weak correlation between the gene array and SILAC ratios highlighted the relevance of quantitative proteomic approaches to estimate the expression of proteins of interest, in concordance with previous reports. There were missing data between both techniques because not all the coding Gomisin-D products of the genes measured by the early version of the Affymetrix oligonucleotide array were detected by SILAC. Similarly, genes coding for the 831 proteins identified by SILAC duplicates were not included among the probes contained in the commercial U133 oligonucleotide array. Availability of both transcript and protein expression levels could also be utilized to uncover potential regulatory mechanisms modifying translation or protein degradation. Immunoblotting validation was closely correlated to the SILAC results, and also served to validate candidates identified in oligonucleotide arrays. Cul3 was selected from the top over-expressed candidates in T24T not previously characterized in bladder cancer for which we had available reagents for further studies. Cul3 was differentially expressed in T24T using three different methodologies: SILAC, gene arrays and immunoblotting. Cul3 is one of the four members of the cullin protein family. It belongs to the core component of multiple ubiquitin-protein ligase complexes that mediate the ubiquitination and subsequent proteasomal degradation of their target proteins. Cul3 acts as a scaffolding protein in a heterodimeric complex playing a central role in the specificity of polyubiquitinization of these proteins, positioning the substrate and the ubiquitin-conjugating enzyme. Although the full list of targets whose ubiquitination and degradation is mediated by Cul3 remains unknown, cancer-related proteins reported include cyclin E, or Rho, among others. In concordance with the interaction network shown in Figure S3, it could be proposed that Cul3 would be involved in the proteasomal degradation of adhesion associated cytoskeletal proteins such as filamin A, ezrin, caveolin1 or moesin. Indeed, the expression of these proteins increased upon Cul3 silencing, observations highlighting the impact of Cul3 expression not only on the aggressive phenotype of T24T shown by functional assays, but also modifying the expression of other proteins identified by SILAC. It remains 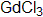 to be characterized whether Cul3 might be directly involved in the proteasomal degradation of cytoskeleton proteins, potentially regulating the migration and invasive aggressiveness properties of T24T cells.
to be characterized whether Cul3 might be directly involved in the proteasomal degradation of cytoskeleton proteins, potentially regulating the migration and invasive aggressiveness properties of T24T cells.
In contrast when using CSF biomarkers to identify at-risk individuals in the asymptom
Individuals with MCI retain relatively high cognitive function, and slowing or arresting the disease in this population offers immense benefits. To explore sample size requirements for clinical trials aimed at this population, we examined enrichment strategies based on CSF and MRI biomarkers to identify MCI individuals who are most likely to experience decline over the course of a clinical trial, and examined the relative ability of subregional and whole brain volume MRI outcome measures to enable further sample size reductions. To assess the relative powers for outcome measures and enrichment choices, we performed statistical significance testing for multiple pair-wise comparisons of outcome measures for different enrichment strategies, and for multiple pair-wise comparisons of enrichment strategies for different outcome measures. There is, however, growing concern that by the time individuals experience noticeable cognitive impairment and brain atrophy, therapies may be too late to stop the neurodegenerative cascade. Thus, preventive trials focused on asymptomatic individuals with biomarker evidence of AD pathology �C and who therefore may be in a preclinical phase of the disorder �C are being considered. To determine the feasibility of such trials, we also assessed rate of clinical decline and regional brain atrophy in cognitively healthy individuals who are likely to be in a presymptomatic stage of AD, based on CSF biomarkers. We considered HCs with CSF evidence of both amyloid and tau pathology as those most likely at risk for developing AD since prior studies have shown that CSF Ab is associated with elevated Tulathromycin B entorhinal cortex atrophy rate and elevated clinical decline only in the presence of elevated CSF ptau. We calculated sample sizes based on the observed rates of change in the HC group that tested positive for both measures, relative to the control group of stable HCs who tested negative for CSF Ab. We used baseline MRI measures to stratify MCI participants into high and low risk groups, as previously described in detail. Briefly, in prior work, we performed a discriminant analysis using cortical and subcortical ROIs to differentiate ADNI’s HC from AD participants. We then applied the resulting model, which incorporated measures of atrophy from medial and lateral temporal areas, retrosplenial cortex, and orbitofrontal areas) to MCI participants, classifying them into those whose atrophy 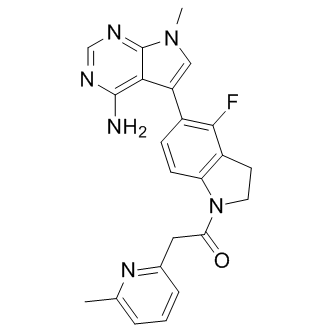 in these regions more strongly resembled that found in the AD group or that found in the HC group. Methodological bias in image registration, leading to artifactually elevated effect sizes and reduced sample size estimates, remains a concern in the structural neuroimaging literature, especially given recent reporting on earlier methodology and results known to be strongly biased, and recent reports citing follow-up methodology and results that are ostensibly LOUREIRIN-B corrected for bias but in fact, as shown in, remain significantly biased. Several robust approaches to reducing or eliminating bias have been developed. Our explicitly inverseconsistent approach essentially eliminates potential bias by combining forward and reverse image registrations, and has been assessed vis-a`-vis other approaches. Here we show that stratifying MCI participants into dichotomized categories with respect to established AD biomarkers results in subgroups of participants with different rates of clinical decline and brain atrophy, and correspondingly different potentially treatable effect sizes that can be leveraged to increase the efficiency of clinical trials. We further show that power for detecting change due to disease progression varies by outcome measure, so that the most powerful outcome measure-enrichment strategy combination dramatically enhances the ability to detect therapeutic effects of investigational disease-altering treatments.
in these regions more strongly resembled that found in the AD group or that found in the HC group. Methodological bias in image registration, leading to artifactually elevated effect sizes and reduced sample size estimates, remains a concern in the structural neuroimaging literature, especially given recent reporting on earlier methodology and results known to be strongly biased, and recent reports citing follow-up methodology and results that are ostensibly LOUREIRIN-B corrected for bias but in fact, as shown in, remain significantly biased. Several robust approaches to reducing or eliminating bias have been developed. Our explicitly inverseconsistent approach essentially eliminates potential bias by combining forward and reverse image registrations, and has been assessed vis-a`-vis other approaches. Here we show that stratifying MCI participants into dichotomized categories with respect to established AD biomarkers results in subgroups of participants with different rates of clinical decline and brain atrophy, and correspondingly different potentially treatable effect sizes that can be leveraged to increase the efficiency of clinical trials. We further show that power for detecting change due to disease progression varies by outcome measure, so that the most powerful outcome measure-enrichment strategy combination dramatically enhances the ability to detect therapeutic effects of investigational disease-altering treatments.
Integrate our findings which indicate decoupling of glycolysis and the Krebs cycle with elevated lactate
In the aggressive SDHB-PHEOs/PGLs into the related energy metabolism pathways, we evaluated the 4-(Benzyloxy)phenol expression of several glycolysis and OXPHOS genes in the human tumors, and in some cases in normal adrenal medulla. We confirmed an elevated GLUT1 and HK2 mRNA expression in VHL- relative to SDHB-PHEOs/PGLs and put it into perspective to normal adrenal 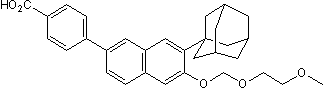 medulla. Elevated HK2 expression is considered a driving force towards a glycolytic phenotype. Increased HK2 expression reportedly leads to enhanced proliferation and resistance to cell death in culture and correlates with poor prognosis in human glioblastoma multiforme. Since VHL-PHEOs/PGLs have a better prognosis than SDHB-PHEOs/PGLs, the difference in HK2 does not seem to contribute to their distinct tumor aggressiveness in these particular tumors. Previously, decreased OXPHOS activity and expression of selected OXPHOS complex subunits have been reported for VHL-derived PHEOs/PGLs. When comparing VHLand SDHB-derived tumor tissues, in the latter we observed the expected decrease in complex II activity, but also an increase in complex III activity. In addition, we found the activity of CS, the first enzyme of the Krebs’ cycle, to be elevated in SDHB-derived tumors. Similar to the data presented by Goffrini et al., our data indicates a selective decrease of complex II activity in SDHBderived PGLs; however there is no evidence for complete disruption of OXPHOS. While we assume that the subunits we evaluated are crucial for the function of the respective complexes, we cannot exclude that they may not reflect the expression levels of other subunits of the same complexes and complex activity. In the present study, findings from the differential expression analysis of the aggressive MTT cells compared to MPC guided our choice for the evaluation of key players in glycolysis and OXPHOS in human SDHB- and VHL-derived PHEOs/PGLs, as a first step in uncovering potential causes for increased tumor aggressiveness in SDHB-related tumors. Our data confirm that both SDHB- and VHL-related PHEOs/PGLs show features of a glycolytic phenotype, while presenting further support for the presence of distinct mechanisms of manifestation of the Warburg effect. In-depth analysis of the roles of elevated LDHA as well as elevated H2O2 production as a possible result of increased SOD2 expression may lead to a better understanding of these tumors and discovery of potential new therapeutic targets for the aggressive phenotype related to SDHB-mutations. Ovarian cancer represents the most Orbifloxacin lethal gynecological malignant disease in the United States. According to the American Cancer Society, if diagnosed at the localized stage, the 5-year survival rate is 94%; however, only 15% of all cases are detected at this stage. The majority of cases of ovarian cancer are diagnosed with distant metastases. For these women the 5-year survival rate is 28%, therefore determining the unique genetic programming that drives ovarian cancer progression is key in diagnosing and treating this disease. We previously identified ARID3B as a target of miR-125a, a microRNA that is under expressed in ovarian cancer. However, the function of ARID3B is relatively unknown. ARID3B belongs to the ARID family of proteins. The ARID family of transcriptional regulators is a conserved group of DNA binding proteins that regulates gene expression. ARID proteins harbor a distinctive DNA-binding domain, the AT-rich interactive domain. Proteins of this family have been implicated in regulation of cell cycle, gene expression, differentiation, embryonic development, chromatin remodeling and transcriptional regulation. Thus, ARID3B could be key regulator in ARID3A function by regulating cellular localization in B cells. Since ARID3B is expressed more broadly than ARID3A it likely has other functions than its regulation of ARID3A.
medulla. Elevated HK2 expression is considered a driving force towards a glycolytic phenotype. Increased HK2 expression reportedly leads to enhanced proliferation and resistance to cell death in culture and correlates with poor prognosis in human glioblastoma multiforme. Since VHL-PHEOs/PGLs have a better prognosis than SDHB-PHEOs/PGLs, the difference in HK2 does not seem to contribute to their distinct tumor aggressiveness in these particular tumors. Previously, decreased OXPHOS activity and expression of selected OXPHOS complex subunits have been reported for VHL-derived PHEOs/PGLs. When comparing VHLand SDHB-derived tumor tissues, in the latter we observed the expected decrease in complex II activity, but also an increase in complex III activity. In addition, we found the activity of CS, the first enzyme of the Krebs’ cycle, to be elevated in SDHB-derived tumors. Similar to the data presented by Goffrini et al., our data indicates a selective decrease of complex II activity in SDHBderived PGLs; however there is no evidence for complete disruption of OXPHOS. While we assume that the subunits we evaluated are crucial for the function of the respective complexes, we cannot exclude that they may not reflect the expression levels of other subunits of the same complexes and complex activity. In the present study, findings from the differential expression analysis of the aggressive MTT cells compared to MPC guided our choice for the evaluation of key players in glycolysis and OXPHOS in human SDHB- and VHL-derived PHEOs/PGLs, as a first step in uncovering potential causes for increased tumor aggressiveness in SDHB-related tumors. Our data confirm that both SDHB- and VHL-related PHEOs/PGLs show features of a glycolytic phenotype, while presenting further support for the presence of distinct mechanisms of manifestation of the Warburg effect. In-depth analysis of the roles of elevated LDHA as well as elevated H2O2 production as a possible result of increased SOD2 expression may lead to a better understanding of these tumors and discovery of potential new therapeutic targets for the aggressive phenotype related to SDHB-mutations. Ovarian cancer represents the most Orbifloxacin lethal gynecological malignant disease in the United States. According to the American Cancer Society, if diagnosed at the localized stage, the 5-year survival rate is 94%; however, only 15% of all cases are detected at this stage. The majority of cases of ovarian cancer are diagnosed with distant metastases. For these women the 5-year survival rate is 28%, therefore determining the unique genetic programming that drives ovarian cancer progression is key in diagnosing and treating this disease. We previously identified ARID3B as a target of miR-125a, a microRNA that is under expressed in ovarian cancer. However, the function of ARID3B is relatively unknown. ARID3B belongs to the ARID family of proteins. The ARID family of transcriptional regulators is a conserved group of DNA binding proteins that regulates gene expression. ARID proteins harbor a distinctive DNA-binding domain, the AT-rich interactive domain. Proteins of this family have been implicated in regulation of cell cycle, gene expression, differentiation, embryonic development, chromatin remodeling and transcriptional regulation. Thus, ARID3B could be key regulator in ARID3A function by regulating cellular localization in B cells. Since ARID3B is expressed more broadly than ARID3A it likely has other functions than its regulation of ARID3A.
Candidate for vaccination against tularemia due to its wild-type nature and the obvious morbidity observed following vaccination
Specifically, F344 rats vaccinated i.t. with 105 CFU U112 in this study were visibly stressed and ill for 7�C10 days following immunization, with symptoms including,10% weight loss, ruffled fur, hunched posture, and periorbital porphyrin production. Such severe morbidity in immunocompetent hosts would likely prevent administration of U112 to immunocompromised individuals. In contrast, vaccination with a hundred-fold higher dose of U112DiglB caused no adverse effects or visible morbidity to rats, and yet this mutant was still able to induce antigen-specific cellular and humoral responses which generated protection against subsequent SCHU S4 challenge. It is likely that booster doses of this mutant strain would increase the degree of protective efficacy. These results collectively suggest the feasibility of developing targeted oral-based attenuated mutant vaccine strains for immunization against F. tularensis and provide impetus for further refinement of novicida-based vaccines, given the ease of its genetic manipulation. Furthermore, M-cells have distinctive morphological features such as a poorly organized brush border, irregular microvilli, and a thin glycocalyx suggesting that they do not play a role in intestinal digestion or absorption. Importantly, M-cells can serve as antigen sampling sites and contain a distinct basal invagination in which live and non-replicating pathogens are presented to lymphocytes, dendritic cells, and macrophages. Synthesis of recombinant extracellular proteins in the human 293 embryonic kidney  cell expression system enables appropriate Mechlorethamine hydrochloride folding and post-translational modifications, thus generating secreted proteins that are functional and structurally similar to the native molecules. To purify the recombinant protein, a common technique includes the addition of a poly-histidine tag at either terminus of the protein; this small tag does typically not alter protein conformation and the imidazole functional group on histidine residues allows for coordination with divalent metal ions and thus purification by immobilized metal affinity chromatography. The histidine-tagged protein binds to nickel and other transition metals immobilized on either an imminodiacetic acid or nitrilo-tri-acetic acid modified chromatography column with high affinity, whereas protein contaminants without the histidine-tag bind weakly or not at all. Histidine-tagged proteins are eluted with imidazole in the range of 20�C200 mM, which competitively displaces proteins bound to the immobilized metal ions. This scheme is widely used to generate numerous secreted proteins including stroma proteins, basement membrane proteins, matricellular proteins, blood proteins and signaling molecules. The Catharanthine sulfate purity of the recombinant protein is typically assessed by SDS-PAGE, Western blotting and mass spectrometry. However, this quality control may be insufficient when the objective of the study is to investigate cell signaling pathways regulated by the protein of interest. In this study, we tested the hypothesis that potent signaling molecules of the TGF-b superfamily are co-purified in this general purification scheme using extracellular fibrillin-1 as an example. If this is the case, it would have important consequences for the quality control of purification schemes and for the design of experiments using recombinant secreted proteins produced in this fashion. Fibrillin-1 is a 350 kDa glycoprotein that multimerizes to form microfibril suprastructures in elastic and non-elastic tissues. Due to its modular domain structure, fragments of fibrillins can be produced and purified conveniently as correctly folded proteins using the HEK293 expression system. Direct interactions between TGF-b and fibrillin-1 have not been documented. However, other members of the TGF-b superfamily including GDF-5 can interact directly.
cell expression system enables appropriate Mechlorethamine hydrochloride folding and post-translational modifications, thus generating secreted proteins that are functional and structurally similar to the native molecules. To purify the recombinant protein, a common technique includes the addition of a poly-histidine tag at either terminus of the protein; this small tag does typically not alter protein conformation and the imidazole functional group on histidine residues allows for coordination with divalent metal ions and thus purification by immobilized metal affinity chromatography. The histidine-tagged protein binds to nickel and other transition metals immobilized on either an imminodiacetic acid or nitrilo-tri-acetic acid modified chromatography column with high affinity, whereas protein contaminants without the histidine-tag bind weakly or not at all. Histidine-tagged proteins are eluted with imidazole in the range of 20�C200 mM, which competitively displaces proteins bound to the immobilized metal ions. This scheme is widely used to generate numerous secreted proteins including stroma proteins, basement membrane proteins, matricellular proteins, blood proteins and signaling molecules. The Catharanthine sulfate purity of the recombinant protein is typically assessed by SDS-PAGE, Western blotting and mass spectrometry. However, this quality control may be insufficient when the objective of the study is to investigate cell signaling pathways regulated by the protein of interest. In this study, we tested the hypothesis that potent signaling molecules of the TGF-b superfamily are co-purified in this general purification scheme using extracellular fibrillin-1 as an example. If this is the case, it would have important consequences for the quality control of purification schemes and for the design of experiments using recombinant secreted proteins produced in this fashion. Fibrillin-1 is a 350 kDa glycoprotein that multimerizes to form microfibril suprastructures in elastic and non-elastic tissues. Due to its modular domain structure, fragments of fibrillins can be produced and purified conveniently as correctly folded proteins using the HEK293 expression system. Direct interactions between TGF-b and fibrillin-1 have not been documented. However, other members of the TGF-b superfamily including GDF-5 can interact directly.