An increased mRNA expression level of Grin1, and a trend to an increased generation of nitrites and a negative correlation of those levels to performance of arsenic exposed mice in at least one of the behavioral tasks. Direct in vivo toxic effect of arsenic and metal mixtures on astrocytes with apoptotic cell death and increased ROS generation were demonstrated recently, and an increased Ca2+ release was suggested as an explanation. Since NMDA receptor is directly involved in calcium homeostasis, sustained up-regulation of Grin1, and therefore a possible disruption of the homeostasis, instigated by arsenic exposure during the embryonic life may provide, at least in part, a molecular mechanism for arsenic neurotoxic effects. While the data from our study does not allow correlation of impaired learning in adult mice to changes in expression level and histone modifications, overall the results are urging an association that should be examined in the future with a goal to Folinic acid calcium salt pentahydrate reveal genomewide, and in an unbiased way, changes in expression of genes that may be critical for learning, memory and synaptic plasticity. In recent years arsenic toxicity and its long term effects on human health have been linked to changes in the epigenome. Arsenic is the only in the group of toxic elements that causes changes in all three epigenetic marks �C DNA methylation, histone modifications and expression of noncoding RNAs. Although the results from different studies have not been consistent, changes in DNA methylation are usually linked to arsenic methylation, which is the normal, physiologically induced, pathway for arsenic detoxification in rodents and humans. The sequestration of CH3 groups by arsenic in the detoxification pathway is considered, therefore, one possible molecular mechanism for global and gene-specific DNA hypomethylation, causing changes in the transcriptional activity of genes implicated in the risk for development of certain diseases. It is difficult to reconcile this hypothesis with the results of some in vivo studies, as well as results from epidemiological studies, where DNA hypermethylation has been reported. The methylation pattern and level of methylation on specific lysine residues in the N-terminal histone tails also change in response to arsenic exposure. However, similar to the changes in DNA methylation pattern, a molecular mechanism explaining concurrent increased and decreased methylation of different lysine residues in the animal models, or humans has not been suggested. The first report that arsenic 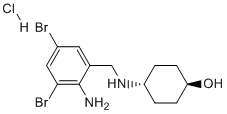 exposure causes a severe reduction in LOUREIRIN-B acetylation of core histones was published in 1983. At this time no clear biological function was assigned to post-translational histone modification. The results with cultured Drosophila cells suggested that the effect of sodium arsenite may be mediated through the activation of heat shock proteins. More recently changes in histone H3 acetylation, and reduced acetylation of H4K16 residue were demonstrated in arsenic treated cultured urothelial and endothelial cells. Elevated histone acetylation responsible for up-regulation of genes involved in apoptosis and stress response has also been reported. The increased global histone acetylation as a result of arsenic exposure in those and other in vitro studies was ascribed to HDAC inhibition. Globally reduced H3K9 acetylation was reported in peripheral mononuclear cells.
exposure causes a severe reduction in LOUREIRIN-B acetylation of core histones was published in 1983. At this time no clear biological function was assigned to post-translational histone modification. The results with cultured Drosophila cells suggested that the effect of sodium arsenite may be mediated through the activation of heat shock proteins. More recently changes in histone H3 acetylation, and reduced acetylation of H4K16 residue were demonstrated in arsenic treated cultured urothelial and endothelial cells. Elevated histone acetylation responsible for up-regulation of genes involved in apoptosis and stress response has also been reported. The increased global histone acetylation as a result of arsenic exposure in those and other in vitro studies was ascribed to HDAC inhibition. Globally reduced H3K9 acetylation was reported in peripheral mononuclear cells.