Desikan et al observed that Lomitapide Mesylate atrophy rate in entorhinal cortex was associated with CSF Ab only in the presence of ptau. Dickerson et al showed that a baseline MRI signature for AD developed in a non-ADNI cohort that was predictive of subsequent clinical decline in HCs was also associated with decreased CSF Ab in HCs. Note that care must be taken when Chlorhexidine hydrochloride comparing results based on PiB, which binds to the neuritic though not diffuse amyloid plaques, and CSF Ab for three reasons: the CSF Ab values are amyloid monomer concentrations, whereas PiB values reflect density of plaques composed of amyloid fibrils; CSF Ab is a global, not a local or regional measure of amyloid; they are not correlates, but rather have different distributions with age, as shown in. Nevertheless, in the current study, a significantly elevated atrophy rate for CSF Ab+ HCs relative to CSF Ab�C HCs was observed only in the isthmus cingulate. Atrophy rate in the parahippocampal gyrus and amygdala was significantly elevated in those additionally testing positive for ptau. The small difference in atrophy rates and rates of clinical decline observed here between HCs testing positive for CSF biomarkers and those testing negative imply that clinical trials, even if of longer duration than the typical 18 to 24 months, will lack power to detect treatment effects using currently available clinical or structural outcome measures. This conclusion is seemingly at odds with the results of a recent study by Schott and colleagues which 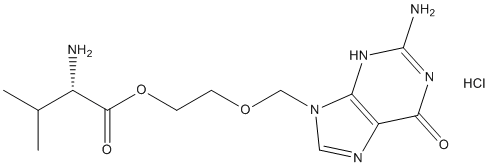 reported that brain atrophy may be a useful outcome measure in preventive trials. In that study ADNI’s HCs were categorized with respect to CSF Ab, using the same cutoff threshold applied here, and sample sizes estimated based on rate of atrophy of whole brain, hippocampus, and ventricles, using baseline and 12-month follow-up MRIs only; whole brain atrophy rate was calculated using the KN-BSI method, HMAPS with BSI was used for the hippocampus, and BSI was used for the ventricles. Results showed that for a treatment effect reported to be equal to 48% of a disease effect calculated from rates of change in 40 Ab+ HCs relative to rates of change in 65 Ab�C HCs, sample size of 141 participants per arm for whole brain atrophy as the outcome measure and 467 participants per arm for hippocampal atrophy as the outcome measure would provide 80% power at a significance of 0.05. However, few clinical trials are powered on the basis of such a large effect size; most studies estimate sample sizes to provide sufficient power to detect a slowing in the disease-related rate of decline of 20% or 25% as we have done here. Scaling Schott and colleagues’ results to an effect size of 25% slowing in disease-related atrophy, to enable comparison with this and prior studies, yields sample size estimates of 500 participants per arm for whole brain atrophy as an outcome, and 1722 participants per arm for hippocampal atrophy as an outcome. Though the large sample size, and large upper confidence interval, renders hippocampal atrophy rate unsuitable for use as an outcome measure in a preclinical treatment trial, this analysis suggests that whole brain atrophy could be a feasibly outcome measure in a large preclinical trial. However, there is another important difference in the analysis methods that must be considered. Schott and colleagues estimated sample sizes using two timepoints only: baseline and a single followup at 12 months. More reliable estimates of atrophy rates and associated variances, and sample sizes derived from these, would come from using all available followup timepoints �C of which there are up to four covering up to 36 months per HC participant as we have done here.
reported that brain atrophy may be a useful outcome measure in preventive trials. In that study ADNI’s HCs were categorized with respect to CSF Ab, using the same cutoff threshold applied here, and sample sizes estimated based on rate of atrophy of whole brain, hippocampus, and ventricles, using baseline and 12-month follow-up MRIs only; whole brain atrophy rate was calculated using the KN-BSI method, HMAPS with BSI was used for the hippocampus, and BSI was used for the ventricles. Results showed that for a treatment effect reported to be equal to 48% of a disease effect calculated from rates of change in 40 Ab+ HCs relative to rates of change in 65 Ab�C HCs, sample size of 141 participants per arm for whole brain atrophy as the outcome measure and 467 participants per arm for hippocampal atrophy as the outcome measure would provide 80% power at a significance of 0.05. However, few clinical trials are powered on the basis of such a large effect size; most studies estimate sample sizes to provide sufficient power to detect a slowing in the disease-related rate of decline of 20% or 25% as we have done here. Scaling Schott and colleagues’ results to an effect size of 25% slowing in disease-related atrophy, to enable comparison with this and prior studies, yields sample size estimates of 500 participants per arm for whole brain atrophy as an outcome, and 1722 participants per arm for hippocampal atrophy as an outcome. Though the large sample size, and large upper confidence interval, renders hippocampal atrophy rate unsuitable for use as an outcome measure in a preclinical treatment trial, this analysis suggests that whole brain atrophy could be a feasibly outcome measure in a large preclinical trial. However, there is another important difference in the analysis methods that must be considered. Schott and colleagues estimated sample sizes using two timepoints only: baseline and a single followup at 12 months. More reliable estimates of atrophy rates and associated variances, and sample sizes derived from these, would come from using all available followup timepoints �C of which there are up to four covering up to 36 months per HC participant as we have done here.