The administration of chemical chaperones that promote protein folding in the ER has been reported to be effective in treating type2 diabetes, which has been shown, in experiments using a mouse model, to be connected to ER stress. We have also shown that a chemical chaperone prevented the development of morphine tolerance caused by ER stress. Our present study suggests that ER chaperones would be promising therapeutic targets in the treatment of chronic neurodegenerative diseases. Chronic thromboembolic pulmonary hypertension is a form of pulmonary hypertension caused by persistent thromboemboli of the pulmonary arteries. It can be treated by pulmonary endarterectomy, provided that the thrombi are surgically accessible. However, CTEPH can be difficult to treat if the thrombi are limited to peripheral pulmonary arteries, or if peripheral vascular remodeling has occurred, similar to the pathobiology in patients with idiopathic pulmonary arterial hypertension. Few patients with acute pulmonary thromboembolism develop CTEPH, but early diagnosis of this rare, refractory disease could improve prognosis. Currently, brain natriuretic peptide is used widely as a biomarker of CTEPH similar to its use for chronic heart failure. It has also been reported that C-reactive protein and heart-type fatty acid-binding protein are biomarkers of CTEPH. PTX3 binds to several bacteria and viruses as pattern 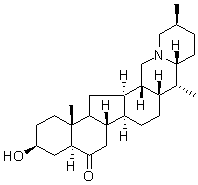 recognition molecule, activate the classical complement pathway by bindings to C1q. Unlike CRP, PTX3 binds to several ligands other than C1q, including fibroblast growth factors -2, Pselectin, and TNF-a-stimulated gene6. PTX3 is also reported to upregulate tissue factor expression in endothelial cells and play a role in thrombogenesis. Any function described above might elevate PTX3 Catharanthine sulfate levels in angina pectoris, acute myocardial infarction, heart failure, Takayasu arteritis, vasculitis, sepsis/systemic inflammatory response syndrome, and other infections. Recently it was shown that PTX3 could reflect any pathophysiological aspect for PAH, especially in patients with connective tissue disease. However, whether PTX3 could be one of the biomarkers in patients with CTEPH remains unclear. The purpose of this study was to investigate whether PTX3 would be a useful biomarker compared to other biomarkers for detecting CTEPH especially with respect to differentiation from clinically stable status after acute episode of PTE. We also examined the relationship between PTX3 levels and pulmonary hemodynamics in patients with CTEPH. To our knowledge, this is the first study to demonstrate elevated plasma PTX3 levels in patients with CTEPH. In addition, we found that PTX3 levels showed mild negative Alprostadil correlation with CO. No significant difference was observed in PTX3 levels before and after successful PEA, or between patients who were or were not treated with PAH-specific therapies. PTX3 levels had better sensitivity than BNP levels, although BNP levels showed considerably stronger correlations with pulmonary hemodynamic parameters. Compared to BNP, PTX3 could identify CTEPH patients with less severe pulmonary hemodynamics. As described above, PTX3 is produced locally in the various cells in response to the stimulation by inflammatory cytokines. On the other hand, BNP is produced mainly in the cardiac ventricles by an increase in stretch and/or pressure. It is thus expected that BNP levels will correlate with hemodynamic parameters, however, BNP levels paradoxically tend to miss the CTEPH patients with preserved right ventricular ejection fraction or low pulmonary arterial pressure. Lang et al. reported that inflammation not only contributes to the pathogenesis of CTEPH, but also to the development of the disease.
recognition molecule, activate the classical complement pathway by bindings to C1q. Unlike CRP, PTX3 binds to several ligands other than C1q, including fibroblast growth factors -2, Pselectin, and TNF-a-stimulated gene6. PTX3 is also reported to upregulate tissue factor expression in endothelial cells and play a role in thrombogenesis. Any function described above might elevate PTX3 Catharanthine sulfate levels in angina pectoris, acute myocardial infarction, heart failure, Takayasu arteritis, vasculitis, sepsis/systemic inflammatory response syndrome, and other infections. Recently it was shown that PTX3 could reflect any pathophysiological aspect for PAH, especially in patients with connective tissue disease. However, whether PTX3 could be one of the biomarkers in patients with CTEPH remains unclear. The purpose of this study was to investigate whether PTX3 would be a useful biomarker compared to other biomarkers for detecting CTEPH especially with respect to differentiation from clinically stable status after acute episode of PTE. We also examined the relationship between PTX3 levels and pulmonary hemodynamics in patients with CTEPH. To our knowledge, this is the first study to demonstrate elevated plasma PTX3 levels in patients with CTEPH. In addition, we found that PTX3 levels showed mild negative Alprostadil correlation with CO. No significant difference was observed in PTX3 levels before and after successful PEA, or between patients who were or were not treated with PAH-specific therapies. PTX3 levels had better sensitivity than BNP levels, although BNP levels showed considerably stronger correlations with pulmonary hemodynamic parameters. Compared to BNP, PTX3 could identify CTEPH patients with less severe pulmonary hemodynamics. As described above, PTX3 is produced locally in the various cells in response to the stimulation by inflammatory cytokines. On the other hand, BNP is produced mainly in the cardiac ventricles by an increase in stretch and/or pressure. It is thus expected that BNP levels will correlate with hemodynamic parameters, however, BNP levels paradoxically tend to miss the CTEPH patients with preserved right ventricular ejection fraction or low pulmonary arterial pressure. Lang et al. reported that inflammation not only contributes to the pathogenesis of CTEPH, but also to the development of the disease.