If cell wall composition influences on the structure and functionality of the tissues, the kidney-shaped gall morphotype on both Baccharis species should present similar immunolabeling for Senegenin pectins and associated proteins. Our objective is to evaluate histochemically and immunocitochemically the presence and distribution of pectins and AGPs during gall development, checking their association with the functional aspects of gall tissue layers. Ergosterol According to this anatomical description, the parenchymatic cells and the secretory ducts hypertrophy as the galls mature. By the time the stimuli for the maintenance of gall tissues stop, the gall naturally opens, and the gall inducer emerges, indicating the existence of transformations in the status of the cell walls, and their components. One of the cell wall components, the pectins, was histochemically detected in all tissue layers of non-galled and galled samples by the positive reaction to ruthenium red and coriphosphine. This second reagent has revealed a slight difference of pectin detection with a weaker fluorescence in the cell walls of the secretory ducts. Ruthenium red has been considered a nonspecific reagent for pectins, or specific for unesterified pectins. The la er author also proposes that ruthenium red stains more intensely the cell walls where deesterification has occurred, i. e., it may stain more intensely low methyl-esterified pectins and less intensely the high methylesterified pectins. The weak staining with ruthenium red in gall samples may be indicative of pectins de-methylesterification during gall maturation and senescence. The histochemical test with coriphosphine was used to confront these results. Coriphosphine does not react with cellulose, but has affinity to esterified pectins. So, the reduced fluorescence observed in the epithelium indicates the presence of low methyl-esterified pectins in the cell walls. Current immunocytochemical analyses labelling of a wide range either of high or low methyl-esterified pectins in non-galled and gall tissues of B. dracunculifolia strongly prove the potential of ruthenium red and coriphosphine for the histochemical detection of overall pectins, but did not corroborate their efficiency for evaluating the degree of pectin esterification. The JIM5 and JIM7 antibodies detected variations on the distribution of pectins in the cell walls of the three plant tissue systems during the development of the kidney-shaped gall of B. dracunculifolia. The variations in the topography and chemical nature of the pectins should be related to the determination of gall shapes, as a network of the domains of the three polysacharides can have the potential to modulate cell wall structure. This modulation was previously tested in the kidney-shaped gall of B. reticularia. Also, the functional aspects of the gall tissue layers, mainly in relation to cell adhesion and expansion, as well 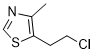 as cell wall porosity, corroborated the assumptions of Knox and McCartney et al.. The degree of methyl-esterification of the homogalacturonans herein analyzed changed from the non-galled tissues towards the senescent galls, which should require the action of pectin methyl esterases or polygalacturonases. Moreover, the de-methyl-esterification can be either randomic or linear, with two distinct biological responses.
as cell wall porosity, corroborated the assumptions of Knox and McCartney et al.. The degree of methyl-esterification of the homogalacturonans herein analyzed changed from the non-galled tissues towards the senescent galls, which should require the action of pectin methyl esterases or polygalacturonases. Moreover, the de-methyl-esterification can be either randomic or linear, with two distinct biological responses.