And FoxO1 and/or FoxO3 are/is considered as apoptosis-regulating gene for the onset of diabetic cardiomyopathy, cardiac 6-gingerol hypertrophy and ischemic heart disease. It has been reported that the upregulation of FoxO1 and FoxO3 appears to disrupt cardiac hypertrophy. In a balloon carotid arterial injury rat model, gene transfer of FoxO3 can inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. The expression of FoxO1 can be stimulated by a1-adrenergic agonists and ultimately lead to apoptotic endothelial cell injury. Considering the possible role of FoxO1 and FoxO3 in the maintenance of vascular homeostasis, in the present study we aimed to investigate the intrinsic association of FoxO1 and FoxO3 with CHD phenotype in Han Chinese. We selected four tagging SNPs of FoxO1 and two tagging SNPs of FoxO3. The frequencies of FoxO1 and FoxO3 were testified in Chinese CHD patients from two different regions. In the present study, we identified four tagging SNPs of FoxO1 and two tagging SNPs of FoxO3 with CHD in two geographically isolated Han Chinese populations. And our data showed that these six investigated SNPs of FoxO1/FoxO3 might not be distributed differently between CHD patients and non-CHD controls in population from Beijing and Harbin. Stratification analysis was carried out to understand the interaction between genetic and other risk factors, and the addition of other risk factors seemed not 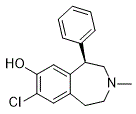 influencing the susceptibility for CHD. These results indicated for the first time that the association of FoxO1/FoxO3 with the risk of CHD was not statistically significant in Han Chinese. How the genetic determinants contribute to CHD has provoked great interest in recent years. These population-based genomewide association studies were trying to identify specific genotypes and alleles responsible for CHD. As is known, FoxOs, acting as important heredity factors in aging, are important regulators involved in the process of oxidative stress, immune surveillance, vascular tone and cardiovascular development. Besides, FoxOs can modulate the metabolic environment by regulating the expressions of specific enzyme and energydependant proteins. Several lines of evidence demonstrated that FoxO1 and FoxO3 were expressed in murine heart and coronary arteries. Altered FoxO1 function in vascular endothelial cells was reported to be responsible for the observed worsening of lesions. FoxO3 can regulate the expression of certain factors in cardiac fibroblasts, such as peroxiredoxin III-a cardioprotectant. Furthermore, FoxO3 is also expressed in endothelial cells, and it can modulate endothelial cell migration and sprouting during vascular development. However, in our genotyping research, neither FoxO1 nor FoxO3 was testified to be associated with CHD. It may be explained with considerations as follows. Atherosclerosis in CHD is often confused with vascular aging. Aged vessels show a number of characteristic pathological processes, many of which are also seen in atherosclerosis. However, by the standards of pathology, arteriosclerosis is divided into three types: atherosclerosis of large and medium-sized arteries, monckeberg medical calcific sclerosis of medium-sized arteries, and arteriolosclerosis. Thus, vascular aging is not included among the three types of arteriosclerosis. Vascular aging is a process characterized by various alternations in a physical environment of cells in vessels. Oxygen free radicals and mitochondrial DNA mutations have been closely associated with vascular aging. It is of interest to note that the incidence of atherosclerosis Ganoderic-acid-G increases with advancing age and aging is a strong risk factor for atherosclerosis.
influencing the susceptibility for CHD. These results indicated for the first time that the association of FoxO1/FoxO3 with the risk of CHD was not statistically significant in Han Chinese. How the genetic determinants contribute to CHD has provoked great interest in recent years. These population-based genomewide association studies were trying to identify specific genotypes and alleles responsible for CHD. As is known, FoxOs, acting as important heredity factors in aging, are important regulators involved in the process of oxidative stress, immune surveillance, vascular tone and cardiovascular development. Besides, FoxOs can modulate the metabolic environment by regulating the expressions of specific enzyme and energydependant proteins. Several lines of evidence demonstrated that FoxO1 and FoxO3 were expressed in murine heart and coronary arteries. Altered FoxO1 function in vascular endothelial cells was reported to be responsible for the observed worsening of lesions. FoxO3 can regulate the expression of certain factors in cardiac fibroblasts, such as peroxiredoxin III-a cardioprotectant. Furthermore, FoxO3 is also expressed in endothelial cells, and it can modulate endothelial cell migration and sprouting during vascular development. However, in our genotyping research, neither FoxO1 nor FoxO3 was testified to be associated with CHD. It may be explained with considerations as follows. Atherosclerosis in CHD is often confused with vascular aging. Aged vessels show a number of characteristic pathological processes, many of which are also seen in atherosclerosis. However, by the standards of pathology, arteriosclerosis is divided into three types: atherosclerosis of large and medium-sized arteries, monckeberg medical calcific sclerosis of medium-sized arteries, and arteriolosclerosis. Thus, vascular aging is not included among the three types of arteriosclerosis. Vascular aging is a process characterized by various alternations in a physical environment of cells in vessels. Oxygen free radicals and mitochondrial DNA mutations have been closely associated with vascular aging. It is of interest to note that the incidence of atherosclerosis Ganoderic-acid-G increases with advancing age and aging is a strong risk factor for atherosclerosis.