The role of let7 has been widely studied in cancer and in stem cell biology, and its potential Atractylenolide-III targets include multiple antiapoptotic and cell proliferation pathways. The insulin signaling network has been known as a cell survival pathway, which is mediated by insulin receptors of two types, insulin receptor and IGF1R. Their activation Gomisin-D phosphorylates Akt and the mammalian target of rapamycin proteins, which induces pro-survival protein synthesis and regulates apoptosis related proteins. The neuroprotective effect of AM let7c is probably mediated by IGF1R up-regulation with decreased apoptotic cell death around the hematoma of 50% compared to controls. Recently the neuroprotective effect of let7f antagomir which is one base pair different from let7c was studied in the cerebral infarction animal model. It showed robust expression of let7f from the ischemic cortex, and the antagonistic sequence of let7f significantly increased IGF1 expression level and decreased the infarct volume. Another study showed the neuroprotective effect of IGF1 and erythropoietin combination treatment via 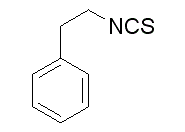 intranasal delivery in the middle cerebral artery occlusion model. Considering several previous studies disclosing the neuroprotective effect of IGF1R activation, it is conceivable to suspect that the IGF1R signal can be a promising therapeutic target in ICH. The possible anti-inflammatory effect of AM let7c treatment is another interesting finding. There exist several lines of evidence suggesting IGF1 signaling relates to inflammation. One study showed that IGF-1 infusion delayed atherosclerotic lesion progression in ApoE-deficient mice by reducing vascular inflammation and inflammatory cytokines. Previous study of let7f in a cerebral infarction model showed let7f is primarily localized in the microglia by in situ hybridization combined with immunohistochemistry, which is related to reduced IGF signaling. It is also probable that there exist other targets of let7c which control the inflammatory cascade after neuronal injury. Recent study found that let7 can function as signaling molecule of Toll-like receptor 7, and contributes to neurodegeneration by activating the innate immune receptor. On the other hand, decreased apoptotic cell death by activation of the IGF1R pathway may have indirectly reduced reactive inflammatory cell recruitment around the hematoma. Future studies focusing on inflammatory cytokine production and immune cell regulation by let7c modulation will help to increase understanding of its role in inflammation modulation. Intranasal delivery has been suggested as a convenient and effective transmission modality for central nervous system acting medications. The proposed route of administration is along the olfactory and trigeminal neural pathways from the nasal mucosa to the brain and the drug enters the perivascular spaces from which it is rapidly dispersed throughout the brain. Not only drugs, but cells, viral vectors and peptides have been successfully delivered to the brain via intranasal administration. It can bypass first-pass metabolism and allow the direct delivery of the drug to the cerebral spinal fluid. Our group administered antagomiR of miR-206 in the Alzheimer’s disease transgenic mouse model, and showed a cognitive enhancing effect by increasing the BDNF. Another group showed enhanced delivery efficiency of IGF1 via the intranasal route compared to the intravenous or intraperitoneal routes in a cerebral infarction model.
intranasal delivery in the middle cerebral artery occlusion model. Considering several previous studies disclosing the neuroprotective effect of IGF1R activation, it is conceivable to suspect that the IGF1R signal can be a promising therapeutic target in ICH. The possible anti-inflammatory effect of AM let7c treatment is another interesting finding. There exist several lines of evidence suggesting IGF1 signaling relates to inflammation. One study showed that IGF-1 infusion delayed atherosclerotic lesion progression in ApoE-deficient mice by reducing vascular inflammation and inflammatory cytokines. Previous study of let7f in a cerebral infarction model showed let7f is primarily localized in the microglia by in situ hybridization combined with immunohistochemistry, which is related to reduced IGF signaling. It is also probable that there exist other targets of let7c which control the inflammatory cascade after neuronal injury. Recent study found that let7 can function as signaling molecule of Toll-like receptor 7, and contributes to neurodegeneration by activating the innate immune receptor. On the other hand, decreased apoptotic cell death by activation of the IGF1R pathway may have indirectly reduced reactive inflammatory cell recruitment around the hematoma. Future studies focusing on inflammatory cytokine production and immune cell regulation by let7c modulation will help to increase understanding of its role in inflammation modulation. Intranasal delivery has been suggested as a convenient and effective transmission modality for central nervous system acting medications. The proposed route of administration is along the olfactory and trigeminal neural pathways from the nasal mucosa to the brain and the drug enters the perivascular spaces from which it is rapidly dispersed throughout the brain. Not only drugs, but cells, viral vectors and peptides have been successfully delivered to the brain via intranasal administration. It can bypass first-pass metabolism and allow the direct delivery of the drug to the cerebral spinal fluid. Our group administered antagomiR of miR-206 in the Alzheimer’s disease transgenic mouse model, and showed a cognitive enhancing effect by increasing the BDNF. Another group showed enhanced delivery efficiency of IGF1 via the intranasal route compared to the intravenous or intraperitoneal routes in a cerebral infarction model.
Month: April 2019
There has been a recent report studying the circulating blood miRNA level in ICH patients
Recently we studied the miRNA expression pattern in a human Alzheimer’s diseasebrain sample and Tg2576 AD transgenic mouse brain, and found that the level of miR-206, which regulates brain derived neurotrophic factor, was markedly increased in AD mice. The inhibition of miR-206 by intranasal antagonistic Ganoderic-acid-F sequence administration increased brain BDNF and improved memory function. We also showed that multiple miRNAs were increased in a mouse stroke model and proved its modulation had neuroprotective potential in the in vitro oxygen glucose deprivation condition. Regarding ICH, there has been one study reporting miRNA expression patterns in animal models, but no study has investigated the therapeutic potential of miRNA modulation. Considering previous messenger RNA expression analysis in ICH animal models and human brains reporting downregulation of cell survival pathways and increased inflammatory gene expression, it is expected that miRNA directed gene modulation could be a feasible therapeutic approach in ICH. In this study we tried to evaluate the miRNA expression pattern in a rat collagenase induced ICH model to understand ICH specific pathophysiology and to discover therapeutic targets by miRNA modulation. Using miRNA microarray and quantitative real-time polymerase chain reaction, we selected candidate miRNA with potential therapeutic effect. Whether its inhibition by counter sequenced miRNA, namely antagomir, has a neuroprotective effect was studied using the in vitro thrombin toxicity model and in vivo ICH models. This study shows the miRNA expression pattern after ICH induction, and the results indicate that up-regulated miRNAs could be a potential therapeutic target in ICH. When let7c was abrogated by its antagonist application, neuronal survival was increased in the in vitro thrombin toxicity model and functional improvement was facilitated after ICH, with reduced apoptotic cell death. The IGF1R expression was influenced by let7c modulation, and the activated cell survival pathway after IGF1R signaling is proposed as a plausible therapeutic mechanism of AM let7c. This study shows the characteristic miRNA expression profile in the ICH model with region-specific elevation. The expression pattern of miRNA  had been previously reported from three different brain hemorrhage models produced by intraventricular autologous blood, lysed blood and thrombin injection. They showed that miR-298 and miR-245 increased in the blood injected ICH model, and that there was a miR-107, miR-200b and miR331-5p increment in the thrombin injection model. The miRNA expression pattern of our study is different from the results of the previous study because the researchers harvested the UNC669 hippocampus for miRNA extraction, although hippocampus is not the principally injured site in either the collagenase induced ICH model or human ICH patient. We evaluated regional differences of miRNA expression after ICH and found that let 7c was upregulated more than four folds in the basal ganglia compared to the contralateral side. Considering that the basal ganglia are one of the most frequent ICH locations in the clinical field, it is rational to suspect a pathophysiologic role of let7c and to study its modulation effect in ICH. Blood degradation product such as thrombin might be important in let7c induction, because let7c expression was also increased in blood injection model, but not in saline injection model.
had been previously reported from three different brain hemorrhage models produced by intraventricular autologous blood, lysed blood and thrombin injection. They showed that miR-298 and miR-245 increased in the blood injected ICH model, and that there was a miR-107, miR-200b and miR331-5p increment in the thrombin injection model. The miRNA expression pattern of our study is different from the results of the previous study because the researchers harvested the UNC669 hippocampus for miRNA extraction, although hippocampus is not the principally injured site in either the collagenase induced ICH model or human ICH patient. We evaluated regional differences of miRNA expression after ICH and found that let 7c was upregulated more than four folds in the basal ganglia compared to the contralateral side. Considering that the basal ganglia are one of the most frequent ICH locations in the clinical field, it is rational to suspect a pathophysiologic role of let7c and to study its modulation effect in ICH. Blood degradation product such as thrombin might be important in let7c induction, because let7c expression was also increased in blood injection model, but not in saline injection model.
Cytoskeletal structures in myocytes and has so far been regarded as muscle-specific
The current study demonstrates to our knowledge for the first time that this protein is also markedly expressed in postnatal CNS tissue and therefore not restricted to muscle tissue. Western-blot Sipeimine analysis and quantitative PCR revealed a transient retinal MLP expression starting at E20, reaching a peak between P7 and P14 and absence in the adult retina. Immunohistochemical analysis confined  MLP expression in cholinergicAC. These neurons are one of at least 26 distinguishable AC subtypes with the ability to produce the neurotransmitter acetylcholine and are involved in the maturation of postnatal retinaas well as motion sensation by the visual apparatus. In rodents, first AC are born at E8/E9, along with retinal ganglion cells, horizontal cells and cone photoreceptors. AC genesis peaks at E16/E17, but proceeds at least until P5/P7. Consistent with the data presented in the current study, cholinergic AC start to express choline acetyltransferaseat around E18 and therefore almost simultaneously to the observed induction of retinal MLP-expression. However, in contrast to the lifelong expression of ChAT, MLP is only expressed throughout the first 3 weeks after birth and 4-Aminohippuric Acid absent in adult AC. During this postnatal time period AC form dendrites, establish two well-separated cholinergic dendritic layers in the IPL and make synapses on RGCs and bipolar cells. This process also timely overlaps with the emergence of spontaneous acetylcholine dependent waves of excitatory activity between P0 and P11, which are important for the formation of synapses and the establishment of neural circuits between retinal neurons. The formation of specific synapses between cholinergic AC and direction-selective RGCs, known to be crucial for motion sensing, occurs in the second postnatal week and is therefore timely correlated with morphologic maturation of AC. Maturation of cholinergic AC is eventually accomplished at postnatal day 15, the time of eye openingand of detected decrease of MLP expression, suggesting a potential functional involvement of this protein in this context. Future studies still have to investigate the potential role of MLP during AC development. To this end the morphology of AC or other retinal cells as well as the visual function of MLP deficient and wild-type mice could be compared with each other to identify potential differences. In muscle tissue MLP is crucially involved in myogenesis and myocyte differentiation, requiring the localization of MLP in the nucleus, where it serves as transcriptional cofactor and modulates the expression of myocyte specific genes. In addition, MLP is also located in the cytoplasm where it might promote the assembly of cytoskeletal proteins along actin-based filaments. As MLP was only detected in the cytoplasm of AC it appears more likely that it is rather involved in the organization of cytoskeleton than regulation of gene expression in these neurons. In this context MLP may support the growth and stratification of dendrites or synapse formation. MLP has been shown to interact with a broad variety of proteins belonging to different functional classes in muscle tissue. In particular, interactions of MLP with aactin, actin-binding proteins like cofilin 2, spectrins or even metabolic enzymes like D-lactate dehydrogenasewere described. Therefore, the role of MLP in cholinergic AC might depend on the interaction with several partners. Further research is required to address these possibilities.
MLP expression in cholinergicAC. These neurons are one of at least 26 distinguishable AC subtypes with the ability to produce the neurotransmitter acetylcholine and are involved in the maturation of postnatal retinaas well as motion sensation by the visual apparatus. In rodents, first AC are born at E8/E9, along with retinal ganglion cells, horizontal cells and cone photoreceptors. AC genesis peaks at E16/E17, but proceeds at least until P5/P7. Consistent with the data presented in the current study, cholinergic AC start to express choline acetyltransferaseat around E18 and therefore almost simultaneously to the observed induction of retinal MLP-expression. However, in contrast to the lifelong expression of ChAT, MLP is only expressed throughout the first 3 weeks after birth and 4-Aminohippuric Acid absent in adult AC. During this postnatal time period AC form dendrites, establish two well-separated cholinergic dendritic layers in the IPL and make synapses on RGCs and bipolar cells. This process also timely overlaps with the emergence of spontaneous acetylcholine dependent waves of excitatory activity between P0 and P11, which are important for the formation of synapses and the establishment of neural circuits between retinal neurons. The formation of specific synapses between cholinergic AC and direction-selective RGCs, known to be crucial for motion sensing, occurs in the second postnatal week and is therefore timely correlated with morphologic maturation of AC. Maturation of cholinergic AC is eventually accomplished at postnatal day 15, the time of eye openingand of detected decrease of MLP expression, suggesting a potential functional involvement of this protein in this context. Future studies still have to investigate the potential role of MLP during AC development. To this end the morphology of AC or other retinal cells as well as the visual function of MLP deficient and wild-type mice could be compared with each other to identify potential differences. In muscle tissue MLP is crucially involved in myogenesis and myocyte differentiation, requiring the localization of MLP in the nucleus, where it serves as transcriptional cofactor and modulates the expression of myocyte specific genes. In addition, MLP is also located in the cytoplasm where it might promote the assembly of cytoskeletal proteins along actin-based filaments. As MLP was only detected in the cytoplasm of AC it appears more likely that it is rather involved in the organization of cytoskeleton than regulation of gene expression in these neurons. In this context MLP may support the growth and stratification of dendrites or synapse formation. MLP has been shown to interact with a broad variety of proteins belonging to different functional classes in muscle tissue. In particular, interactions of MLP with aactin, actin-binding proteins like cofilin 2, spectrins or even metabolic enzymes like D-lactate dehydrogenasewere described. Therefore, the role of MLP in cholinergic AC might depend on the interaction with several partners. Further research is required to address these possibilities.
The final step of autophagy involves the fusion of the autophagosome with the lysosome to form
In RA, synovial fibroblasts and T cells form a costimulatory circuit to perpetuate the inflammation. RASF could inhibit the apoptotic process of T cells and elicit their spontaneous proliferation; the increased inflammatory T cells then in turn induce more robust RASF activation. Cell-cell contact and soluble factors, such as inflammatory cytokines, contribute to this Danshensu interaction. As demonstrated in our study, after TLR ligation, RASF could secrete more inflammatory mediators and express higher levels of surface TSP-1, SDF-1, and IL-15. These factors together with other unknown elements may eventually lead to the amplification of Th1 and Th17 cells. Thus TLRs also contribute to the maintenance and perpetuation of the inflammation in RA. Given the integral roles of TLRs in the initiation, propagation, and perpetuation of the inflammation in RASF as well as T cells, targeting TLRs would be a preferred therapeutic strategy. In fact, several groups have been testing the feasibility of this hypothesis by both animal and human studies. In a serum-transfer model, disease duration was shortened in TLR4 null mice. In the pristane-induced arthritis rat model, TLR3 expression was significantly upregulated during early disease stages. TLR3 agonist stimulation augmented the disease severity, and small interfering RNAtargeting TLR3 in vivo reduced disease severity. The recombinant analogue of chaperonin 10, XToll, targeting TLR4 developed by Cbio Ltd, is now being tested in a phase II clinical trial for RA treatment given by subcutaneous injection. Antibodies targeting TLR2, OPN-305 and OPN-301, have been proved to be able to abrogate spontaneous cytokine release by RASF. DNA-based TLR7/9 antagonist, IMO3100, has been tested in phase I clinical trials and showed promising results for RA. All these prove that targeting TLRs or their activation Ganoderic-acid-G represents an attractive therapeutic option for RA. However, we should notice that there are different types of TLRs in RA as demonstrated in our study. They might interact or work cooperatively with each other during the disease. Targeting one specific TLR might not suffice for RA amelioration. More indepth studies should be taken to further elucidate the pathogenic roles of TLRs in RA and the potential of targeting them for overcoming the chronic and persistent disease. Autophagy is a highly conserved housekeeping pathway that plays a critical role in the removal of aged or damaged intracellular organelles and their delivery to lysosomes for degradation. There are three major autophagic pathways that have been described, microautophagy, chaperone-mediated autophagy, and macroautophagy. Autophagy can be stimulated by a number of events including nutrient deprivation, exposure to pathogens, and oxidative stress. Microautophagy involves  the direct phagocytosis of cytoplasmic elements and subsequent degradation of the elements in the lysosomal lumen. The second major type of autophagy is chaperone-mediated autophagy. Chaperone-mediated autophagy involves the delivery of proteins directly to the lysosome via chaperones such as Hsc70. The third and the best-characterized autophagic pathway is macroautophagy. Autophagy follows a specific series of events, starting with initiation of formation of the phagophore. The phagophore elongates, engulfing a portion of cytoplasm containing the cargo to be degraded, and closes off to form the autophagosome.
the direct phagocytosis of cytoplasmic elements and subsequent degradation of the elements in the lysosomal lumen. The second major type of autophagy is chaperone-mediated autophagy. Chaperone-mediated autophagy involves the delivery of proteins directly to the lysosome via chaperones such as Hsc70. The third and the best-characterized autophagic pathway is macroautophagy. Autophagy follows a specific series of events, starting with initiation of formation of the phagophore. The phagophore elongates, engulfing a portion of cytoplasm containing the cargo to be degraded, and closes off to form the autophagosome.
The proteosome is inhibited the gathered cargo is then degraded by lysosomal hydrolases
It has also been described the role of p53 tumor suppressor protein in regulating autophagy. In fact, depending on the p53 location, this protein exerts  distinct and important function. Regarding this dual role, nuclear p53 acts as a transcription factor to stimulate both DRAM1 and Sestrin 2, which in turns switch on autophagy. In contrast, cytoplasmic p53 inhibits autophagy. In order to induce autophagy, p53 is finally degraded through proteosomes. Efficient autophagy or autophagocytosis is dependent on an equilibrium between the formation and elimination of autophagosomes; thus, a deficit in any part of this pathway will cause autophagic dysfunction. Autophagy plays a role in aging and agerelated diseases. Recent studies show that autophagy and changes in lysosomal activity are associated with both retinal aging and age-related macular degeneration. During autophagy, the cytosolic form of microtubule-associated protein 1A/1B- light chain 3, LC3-I is processed and recruited to the phagophore where it undergoes site specific proteolysis and lipidation near the C terminus to form LC3-II. Autophagy can be stimulated by a change of environmental conditions such as nutrient deprivation, various hormonal stimuli, and other factors.. Recent studies have shed light on the importance of autophagy in both normal development,tissue remodeling, and in pathological conditions. Autophagy does not only serve to protect cells, but it may also contribute to cell damage. Induction of autophagy serves as an early stress response in axonal dystrophy and may participate in the remodeling of axon structures. Autophagy also plays a major role in ocular patho-physiology. Morphological signs of autophagy have been described in the developing retina, participating in Eleutheroside-E programmed cell death. Molecular aging does occur in the trabecular meshwork, the main regulator of aqueous humor outflow. Oxidative damage in TM occur in primary open angle glaucomaand is strictly related with intraocular pressure increase and visual field damage. Salvianolic-acid-B Indeed, dysfunction of the TM increases the resistance of the outflow pathway and induces an increase in intraocular pressure. The number of TM cells decreases with agingthe histopathologic findings of primary open-angle glaucoma tissue being similar to those of aged tissue. Reactive oxygen speciesdamage TM cells, induce apoptosis, and promote cellular aging in this tissue. Oxidative damage and the associated mitochondrial dysfunction may result in energy depletion, accumulation of cytotoxic mediators and cell death. TM cells of the human eye have been suggested as the proper model system for the study of the cellular aging. It was reported that the number of TM cells decreases due to tissue damage induced by oxidative stressand senescence as occurring in glaucoma. Oxidative stress responses, including metabolites redistribution alter the acetylation status of proteins, in human cells with mitochondrial dysfunction and in aging. On the other hand, autophagy and mitophagy eliminate defective mitochondria and serve as a scavenger and apoptosis defender of cells in response to oxidative stress during aging. These scenarios mediate the adaptation of cells to respond to aging and age-related disorders for survival. In the natural course of aging, the homeostasis in the network of oxidative stress responses is disturbed by a progressive increase in the intracellular level of the ROS generated by defective mitochondria.
distinct and important function. Regarding this dual role, nuclear p53 acts as a transcription factor to stimulate both DRAM1 and Sestrin 2, which in turns switch on autophagy. In contrast, cytoplasmic p53 inhibits autophagy. In order to induce autophagy, p53 is finally degraded through proteosomes. Efficient autophagy or autophagocytosis is dependent on an equilibrium between the formation and elimination of autophagosomes; thus, a deficit in any part of this pathway will cause autophagic dysfunction. Autophagy plays a role in aging and agerelated diseases. Recent studies show that autophagy and changes in lysosomal activity are associated with both retinal aging and age-related macular degeneration. During autophagy, the cytosolic form of microtubule-associated protein 1A/1B- light chain 3, LC3-I is processed and recruited to the phagophore where it undergoes site specific proteolysis and lipidation near the C terminus to form LC3-II. Autophagy can be stimulated by a change of environmental conditions such as nutrient deprivation, various hormonal stimuli, and other factors.. Recent studies have shed light on the importance of autophagy in both normal development,tissue remodeling, and in pathological conditions. Autophagy does not only serve to protect cells, but it may also contribute to cell damage. Induction of autophagy serves as an early stress response in axonal dystrophy and may participate in the remodeling of axon structures. Autophagy also plays a major role in ocular patho-physiology. Morphological signs of autophagy have been described in the developing retina, participating in Eleutheroside-E programmed cell death. Molecular aging does occur in the trabecular meshwork, the main regulator of aqueous humor outflow. Oxidative damage in TM occur in primary open angle glaucomaand is strictly related with intraocular pressure increase and visual field damage. Salvianolic-acid-B Indeed, dysfunction of the TM increases the resistance of the outflow pathway and induces an increase in intraocular pressure. The number of TM cells decreases with agingthe histopathologic findings of primary open-angle glaucoma tissue being similar to those of aged tissue. Reactive oxygen speciesdamage TM cells, induce apoptosis, and promote cellular aging in this tissue. Oxidative damage and the associated mitochondrial dysfunction may result in energy depletion, accumulation of cytotoxic mediators and cell death. TM cells of the human eye have been suggested as the proper model system for the study of the cellular aging. It was reported that the number of TM cells decreases due to tissue damage induced by oxidative stressand senescence as occurring in glaucoma. Oxidative stress responses, including metabolites redistribution alter the acetylation status of proteins, in human cells with mitochondrial dysfunction and in aging. On the other hand, autophagy and mitophagy eliminate defective mitochondria and serve as a scavenger and apoptosis defender of cells in response to oxidative stress during aging. These scenarios mediate the adaptation of cells to respond to aging and age-related disorders for survival. In the natural course of aging, the homeostasis in the network of oxidative stress responses is disturbed by a progressive increase in the intracellular level of the ROS generated by defective mitochondria.