responsible for the prostaglandins essential for normal mucosal physiology in gut. As no gastrointestinal toxicity data were collected in this study, whether these phytochemicals cause gastrointestinal bleeding is still unknown and further study in these areas is required. Rhabdomyosarcoma is the most common soft tissue sarcoma in childhood, accounting for about 3% of all childhood tumors. This strategy has improved the CPI-613 survival rate for patients with localized disease to 70% albeit with significant toxicity. Despite aggressive multimodal therapy, high risk patients continue to have a poor prognosis with overall survival rates of 20�C30%. Therefore, there remains a great need for new therapies targeting the molecular pathways which are found to be altered in RMS. RMS tumors typically arise from skeletal muscle and are categorized as either of the alveolar or embryonal subtype based on their histology. ARMS tumors are driven by a translocation involving chromosome 2 or 1 with chromosome 13, resulting in the production of the fusion oncogene PAX3- or PAX7-FOXO1, respectively. In contrast, ERMS tumors commonly harbor loss of heterozygosity at 11p15.5 as well as point mutations in TP53, NRAS, KRAS, HRAS, PIK3CA and FGFR4 genes. Fibroblast Growth Factor Receptor 4, a FGF receptor family member, is a receptor tyrosine kinase that is implicated in the differentiation of myoblasts into skeletal muscle and muscle regeneration after injury. Highlighting a potential role in RMS, early microarray studies of RMS cell lines and tumors showed massive overexpression of FGFR4 and subsequent work showed that FGFR4 is a direct transcriptional target of the PAX3-FOXO1 fusion protein. Of note, recent sequencing studies identified activating mutations specific to FGFR4 in 7.5% of RMS tumors. These mutations occur at amino acid 535 and 550 of the kinase domain and promote tumor growth and metastasis in vivo by constitutively activating FGFR4. These reports emphasize the importance of FGFR4 in RMS and establish this cell surface tyrosine kinase receptor as a candidate target for RMS therapy. Ponatinib is an orally administered 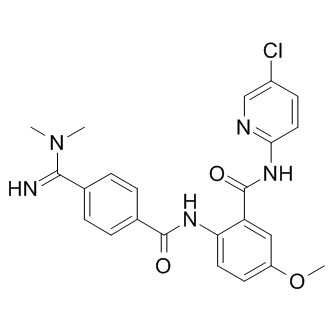 tyrosine kinase inhibitor that was initially developed as an inhibitor for native and mutant forms of BCR-ABL. Recently, this therapy received accelerated FDA approval for the treatment of adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia and chronic phase, accelerated phase, or blast phase chronic myeloid leukemia who are resistant or intolerant to prior tyrosine kinase inhibitor therapy. The inhibition profile of ponatinib includes several other tyrosine Silmitasertib kinases, including FLT3, SRC, KIT, PDGFR, and FGFR. Of note, ponatinib has been shown to inhibit all four members of the FGFR family with an IC50 of less than 40 nM. Inhibition of FGFR family members by ponatinib has been demonstrated in preclinical models of endometrial cancers with FGFR2 mutations, bladder cancers with FGFR3 mutations, as well as breast, lung, and colon cancer cell lines harboring amplification of the FGFR1 or FGFR2 gene. In this study, a panel of RMS cell lines as well as a Ba/F3 cell line engineered to overexpress FGFR4 were tested for sensitivity to five FGFR tyrosine kinase inhibitors, including AP24534, AZD2171, BIBF1120, TKI258, and PHA739358. Of these, ponatinib was found to be the most potent FGFR4 inhibitor, inhibiting both wild-type and mutated FGFR4 phosphorylation and cell growth. Ponatinib also inhibited growth of tumors expressing mutated FGFR4 in vivo. Therefore, our results indicate that ponatinib is an effective FDA-approved drug which has the potential to treat RMS with overexpressed or mutated FGFR4. Alteration of FGFR4 signaling is a common mechanism of oncogenesis in both fusion positive and fusion negative rhabdomyosarcoma. Thus far, at least three mechanisms have been reported to result in the gain of function of FGFR4 in RMS. First, elevated FGFR4 expression in RMS tumors can be a direct result of the PAX3-FOXO1 fusion oncogene, since FGFR4 was reported to be one of the direct targets of the transcription factor. Secondly, up-regulation of FGFR4 expression in RMS can be achieved through localized gene amplification. Thirdly, 7.5% of primary RMS tumors harbor a damaging missense mutation in the tyrosine kinase domain of FGFR4 which results in a constitutively active signaling molecule. The first two mechanisms result in elevated expression of wild-type FGFR4 in RMS, which is both common and associated with poor outcome.
tyrosine kinase inhibitor that was initially developed as an inhibitor for native and mutant forms of BCR-ABL. Recently, this therapy received accelerated FDA approval for the treatment of adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia and chronic phase, accelerated phase, or blast phase chronic myeloid leukemia who are resistant or intolerant to prior tyrosine kinase inhibitor therapy. The inhibition profile of ponatinib includes several other tyrosine Silmitasertib kinases, including FLT3, SRC, KIT, PDGFR, and FGFR. Of note, ponatinib has been shown to inhibit all four members of the FGFR family with an IC50 of less than 40 nM. Inhibition of FGFR family members by ponatinib has been demonstrated in preclinical models of endometrial cancers with FGFR2 mutations, bladder cancers with FGFR3 mutations, as well as breast, lung, and colon cancer cell lines harboring amplification of the FGFR1 or FGFR2 gene. In this study, a panel of RMS cell lines as well as a Ba/F3 cell line engineered to overexpress FGFR4 were tested for sensitivity to five FGFR tyrosine kinase inhibitors, including AP24534, AZD2171, BIBF1120, TKI258, and PHA739358. Of these, ponatinib was found to be the most potent FGFR4 inhibitor, inhibiting both wild-type and mutated FGFR4 phosphorylation and cell growth. Ponatinib also inhibited growth of tumors expressing mutated FGFR4 in vivo. Therefore, our results indicate that ponatinib is an effective FDA-approved drug which has the potential to treat RMS with overexpressed or mutated FGFR4. Alteration of FGFR4 signaling is a common mechanism of oncogenesis in both fusion positive and fusion negative rhabdomyosarcoma. Thus far, at least three mechanisms have been reported to result in the gain of function of FGFR4 in RMS. First, elevated FGFR4 expression in RMS tumors can be a direct result of the PAX3-FOXO1 fusion oncogene, since FGFR4 was reported to be one of the direct targets of the transcription factor. Secondly, up-regulation of FGFR4 expression in RMS can be achieved through localized gene amplification. Thirdly, 7.5% of primary RMS tumors harbor a damaging missense mutation in the tyrosine kinase domain of FGFR4 which results in a constitutively active signaling molecule. The first two mechanisms result in elevated expression of wild-type FGFR4 in RMS, which is both common and associated with poor outcome.