These alterations ultimately result in cell cycle arrest and apoptosis in melanoma cells. The HSP90 inhibitor ganetespib, with IC50 values,100 nM, exhibited profound antiproliferative activity against a panel of cutaneous melanoma cells including those that carry B-RAF and N-RAS mutations, as well as those with acquired resistance to BRAF inhibition. Ganetespib exerted its antiproliferative activity through induction of cell cycle arrest and apoptosis in association with inhibition of multiple tyrosine receptor kinases, B-RAF, CRAF, and the AKT and Erk1/2 pathways. The PI3K/Akt and MAPK/Erk pathways are critical for melanoma cell growth and survivaland were significantly inhibited by ganetespib. During the course of this study, similar effects of the HSP90 inhibitor XL888 on phosphorylation of Akt and Erk1/2 in melanoma cells have been reported. Inhibition of these pathways may contribute to ganetespib induced growth inhibition and apoptosis as inhibition of these pathways, alone or in combination, BKM120 reduced viability of K008 and K028 cellsand induced apoptosis in melanoma cells. In agreement with previous findings that Akt but not Erk1/2 was a client protein of HSP90, the expression of Akt but not Erk1/2 was reduced by ganetespib. Ganetespib may inhibit Akt phosphorylation through downregulating Akt expression and repress Erk1/2 phosphorylation through downregulating their upstream activating kinases, as MEK and 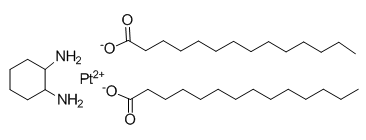 MEKK1 are client proteins of HSP90. Akt and Erk1/2 can be activated by BYL719 signals from multiple tyrosine kinase receptors including EGFR, IGF-1R and c-Met. In agreement with being client proteins of HSP90, the expression of these receptors was decreased by ganetespib. Downregulation of these receptors will also result in inhibition of Akt and Erk1/2 phosphorylation. Thus, ganetespib may inhibit Akt and Erk1/2 activation by targeting multiple cellular signaling processes. Small molecule inhibitors of c-Met, EGFR or IGF-1R reduced viability of K008 and K028 cells that express all these receptors, suggesting that these receptor tyrosine kinases may play a role in survival and growth of these cell lines and their inhibition may be relevant to the anti-melanoma activity of ganetespib. Ganetespib induced cell cycle arrest at G1and/or G2/M phase in cell line dependent manner. Similar cell cycle effects were also observed with XL888. Ganetespib induced cell cycle arrest was associated with upregulation of negative cell cycle regulatorsand/or downregulation of positive regulators. This is in general in line with the roles of these regulators in cell cycle regulation. CDK1, CDK2 and CDK4 have been reported to be chaperoned by HSP90. However, cyclin D1 and cyclin E are not considered to be a client protein for HSP90. The observed downregulation of cyclin D1 may result from downregulation of Akt and Erk1/2 pathways, which control cyclin D1 expression. Downregulation of cyclin E could result from decreased D-type cyclins, as the transcriptional activation of the cyclin E gene depends on the activity of D-type cyclins. The upregulation of the CDK inhibitors p27Kip1 and p21Cip1 could be attributed to inhibition of the Akt and Erk pathways. Despite being reported to be a client protein of HSP90and downregulated in K029 and K033 cells, cyclin B1 was induced by ganetespib in two cell linesthat were arrested at G2/M. Cyclin B1 activity is essential for progression from G2 into M phase. Binding of cyclin B1 to CDK1 allows CDK1 to be activated. The active cyclin B1-CDK1 complex translocates to the nucleus and phosphorylates nuclear substrates. These phosphorylation events are necessary for mitotic onset.
MEKK1 are client proteins of HSP90. Akt and Erk1/2 can be activated by BYL719 signals from multiple tyrosine kinase receptors including EGFR, IGF-1R and c-Met. In agreement with being client proteins of HSP90, the expression of these receptors was decreased by ganetespib. Downregulation of these receptors will also result in inhibition of Akt and Erk1/2 phosphorylation. Thus, ganetespib may inhibit Akt and Erk1/2 activation by targeting multiple cellular signaling processes. Small molecule inhibitors of c-Met, EGFR or IGF-1R reduced viability of K008 and K028 cells that express all these receptors, suggesting that these receptor tyrosine kinases may play a role in survival and growth of these cell lines and their inhibition may be relevant to the anti-melanoma activity of ganetespib. Ganetespib induced cell cycle arrest at G1and/or G2/M phase in cell line dependent manner. Similar cell cycle effects were also observed with XL888. Ganetespib induced cell cycle arrest was associated with upregulation of negative cell cycle regulatorsand/or downregulation of positive regulators. This is in general in line with the roles of these regulators in cell cycle regulation. CDK1, CDK2 and CDK4 have been reported to be chaperoned by HSP90. However, cyclin D1 and cyclin E are not considered to be a client protein for HSP90. The observed downregulation of cyclin D1 may result from downregulation of Akt and Erk1/2 pathways, which control cyclin D1 expression. Downregulation of cyclin E could result from decreased D-type cyclins, as the transcriptional activation of the cyclin E gene depends on the activity of D-type cyclins. The upregulation of the CDK inhibitors p27Kip1 and p21Cip1 could be attributed to inhibition of the Akt and Erk pathways. Despite being reported to be a client protein of HSP90and downregulated in K029 and K033 cells, cyclin B1 was induced by ganetespib in two cell linesthat were arrested at G2/M. Cyclin B1 activity is essential for progression from G2 into M phase. Binding of cyclin B1 to CDK1 allows CDK1 to be activated. The active cyclin B1-CDK1 complex translocates to the nucleus and phosphorylates nuclear substrates. These phosphorylation events are necessary for mitotic onset.