The role of let7 has been widely studied in cancer and in stem cell biology, and its potential Atractylenolide-III targets include multiple antiapoptotic and cell proliferation pathways. The insulin signaling network has been known as a cell survival pathway, which is mediated by insulin receptors of two types, insulin receptor and IGF1R. Their activation Gomisin-D phosphorylates Akt and the mammalian target of rapamycin proteins, which induces pro-survival protein synthesis and regulates apoptosis related proteins. The neuroprotective effect of AM let7c is probably mediated by IGF1R up-regulation with decreased apoptotic cell death around the hematoma of 50% compared to controls. Recently the neuroprotective effect of let7f antagomir which is one base pair different from let7c was studied in the cerebral infarction animal model. It showed robust expression of let7f from the ischemic cortex, and the antagonistic sequence of let7f significantly increased IGF1 expression level and decreased the infarct volume. Another study showed the neuroprotective effect of IGF1 and erythropoietin combination treatment via 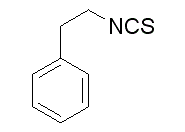 intranasal delivery in the middle cerebral artery occlusion model. Considering several previous studies disclosing the neuroprotective effect of IGF1R activation, it is conceivable to suspect that the IGF1R signal can be a promising therapeutic target in ICH. The possible anti-inflammatory effect of AM let7c treatment is another interesting finding. There exist several lines of evidence suggesting IGF1 signaling relates to inflammation. One study showed that IGF-1 infusion delayed atherosclerotic lesion progression in ApoE-deficient mice by reducing vascular inflammation and inflammatory cytokines. Previous study of let7f in a cerebral infarction model showed let7f is primarily localized in the microglia by in situ hybridization combined with immunohistochemistry, which is related to reduced IGF signaling. It is also probable that there exist other targets of let7c which control the inflammatory cascade after neuronal injury. Recent study found that let7 can function as signaling molecule of Toll-like receptor 7, and contributes to neurodegeneration by activating the innate immune receptor. On the other hand, decreased apoptotic cell death by activation of the IGF1R pathway may have indirectly reduced reactive inflammatory cell recruitment around the hematoma. Future studies focusing on inflammatory cytokine production and immune cell regulation by let7c modulation will help to increase understanding of its role in inflammation modulation. Intranasal delivery has been suggested as a convenient and effective transmission modality for central nervous system acting medications. The proposed route of administration is along the olfactory and trigeminal neural pathways from the nasal mucosa to the brain and the drug enters the perivascular spaces from which it is rapidly dispersed throughout the brain. Not only drugs, but cells, viral vectors and peptides have been successfully delivered to the brain via intranasal administration. It can bypass first-pass metabolism and allow the direct delivery of the drug to the cerebral spinal fluid. Our group administered antagomiR of miR-206 in the Alzheimer’s disease transgenic mouse model, and showed a cognitive enhancing effect by increasing the BDNF. Another group showed enhanced delivery efficiency of IGF1 via the intranasal route compared to the intravenous or intraperitoneal routes in a cerebral infarction model.
intranasal delivery in the middle cerebral artery occlusion model. Considering several previous studies disclosing the neuroprotective effect of IGF1R activation, it is conceivable to suspect that the IGF1R signal can be a promising therapeutic target in ICH. The possible anti-inflammatory effect of AM let7c treatment is another interesting finding. There exist several lines of evidence suggesting IGF1 signaling relates to inflammation. One study showed that IGF-1 infusion delayed atherosclerotic lesion progression in ApoE-deficient mice by reducing vascular inflammation and inflammatory cytokines. Previous study of let7f in a cerebral infarction model showed let7f is primarily localized in the microglia by in situ hybridization combined with immunohistochemistry, which is related to reduced IGF signaling. It is also probable that there exist other targets of let7c which control the inflammatory cascade after neuronal injury. Recent study found that let7 can function as signaling molecule of Toll-like receptor 7, and contributes to neurodegeneration by activating the innate immune receptor. On the other hand, decreased apoptotic cell death by activation of the IGF1R pathway may have indirectly reduced reactive inflammatory cell recruitment around the hematoma. Future studies focusing on inflammatory cytokine production and immune cell regulation by let7c modulation will help to increase understanding of its role in inflammation modulation. Intranasal delivery has been suggested as a convenient and effective transmission modality for central nervous system acting medications. The proposed route of administration is along the olfactory and trigeminal neural pathways from the nasal mucosa to the brain and the drug enters the perivascular spaces from which it is rapidly dispersed throughout the brain. Not only drugs, but cells, viral vectors and peptides have been successfully delivered to the brain via intranasal administration. It can bypass first-pass metabolism and allow the direct delivery of the drug to the cerebral spinal fluid. Our group administered antagomiR of miR-206 in the Alzheimer’s disease transgenic mouse model, and showed a cognitive enhancing effect by increasing the BDNF. Another group showed enhanced delivery efficiency of IGF1 via the intranasal route compared to the intravenous or intraperitoneal routes in a cerebral infarction model.