Recently we studied the miRNA expression pattern in a human Alzheimer’s diseasebrain sample and Tg2576 AD transgenic mouse brain, and found that the level of miR-206, which regulates brain derived neurotrophic factor, was markedly increased in AD mice. The inhibition of miR-206 by intranasal antagonistic Ganoderic-acid-F sequence administration increased brain BDNF and improved memory function. We also showed that multiple miRNAs were increased in a mouse stroke model and proved its modulation had neuroprotective potential in the in vitro oxygen glucose deprivation condition. Regarding ICH, there has been one study reporting miRNA expression patterns in animal models, but no study has investigated the therapeutic potential of miRNA modulation. Considering previous messenger RNA expression analysis in ICH animal models and human brains reporting downregulation of cell survival pathways and increased inflammatory gene expression, it is expected that miRNA directed gene modulation could be a feasible therapeutic approach in ICH. In this study we tried to evaluate the miRNA expression pattern in a rat collagenase induced ICH model to understand ICH specific pathophysiology and to discover therapeutic targets by miRNA modulation. Using miRNA microarray and quantitative real-time polymerase chain reaction, we selected candidate miRNA with potential therapeutic effect. Whether its inhibition by counter sequenced miRNA, namely antagomir, has a neuroprotective effect was studied using the in vitro thrombin toxicity model and in vivo ICH models. This study shows the miRNA expression pattern after ICH induction, and the results indicate that up-regulated miRNAs could be a potential therapeutic target in ICH. When let7c was abrogated by its antagonist application, neuronal survival was increased in the in vitro thrombin toxicity model and functional improvement was facilitated after ICH, with reduced apoptotic cell death. The IGF1R expression was influenced by let7c modulation, and the activated cell survival pathway after IGF1R signaling is proposed as a plausible therapeutic mechanism of AM let7c. This study shows the characteristic miRNA expression profile in the ICH model with region-specific elevation. The expression pattern of miRNA 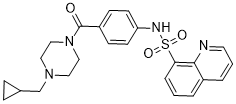 had been previously reported from three different brain hemorrhage models produced by intraventricular autologous blood, lysed blood and thrombin injection. They showed that miR-298 and miR-245 increased in the blood injected ICH model, and that there was a miR-107, miR-200b and miR331-5p increment in the thrombin injection model. The miRNA expression pattern of our study is different from the results of the previous study because the researchers harvested the UNC669 hippocampus for miRNA extraction, although hippocampus is not the principally injured site in either the collagenase induced ICH model or human ICH patient. We evaluated regional differences of miRNA expression after ICH and found that let 7c was upregulated more than four folds in the basal ganglia compared to the contralateral side. Considering that the basal ganglia are one of the most frequent ICH locations in the clinical field, it is rational to suspect a pathophysiologic role of let7c and to study its modulation effect in ICH. Blood degradation product such as thrombin might be important in let7c induction, because let7c expression was also increased in blood injection model, but not in saline injection model.
had been previously reported from three different brain hemorrhage models produced by intraventricular autologous blood, lysed blood and thrombin injection. They showed that miR-298 and miR-245 increased in the blood injected ICH model, and that there was a miR-107, miR-200b and miR331-5p increment in the thrombin injection model. The miRNA expression pattern of our study is different from the results of the previous study because the researchers harvested the UNC669 hippocampus for miRNA extraction, although hippocampus is not the principally injured site in either the collagenase induced ICH model or human ICH patient. We evaluated regional differences of miRNA expression after ICH and found that let 7c was upregulated more than four folds in the basal ganglia compared to the contralateral side. Considering that the basal ganglia are one of the most frequent ICH locations in the clinical field, it is rational to suspect a pathophysiologic role of let7c and to study its modulation effect in ICH. Blood degradation product such as thrombin might be important in let7c induction, because let7c expression was also increased in blood injection model, but not in saline injection model.